The Periodic Table with Standard Colors for Atoms in Molecular Models
The periodic table, displayed with the standard colors used for atoms in molecular models, visually maps elements using a consistent and recognizable color scheme informed by common scientific practice. This color coding helps interpret molecular structures and facilitates communication among chemists and educators, despite the absence of a strict universal standard.
Origins and Source of the Color Scheme
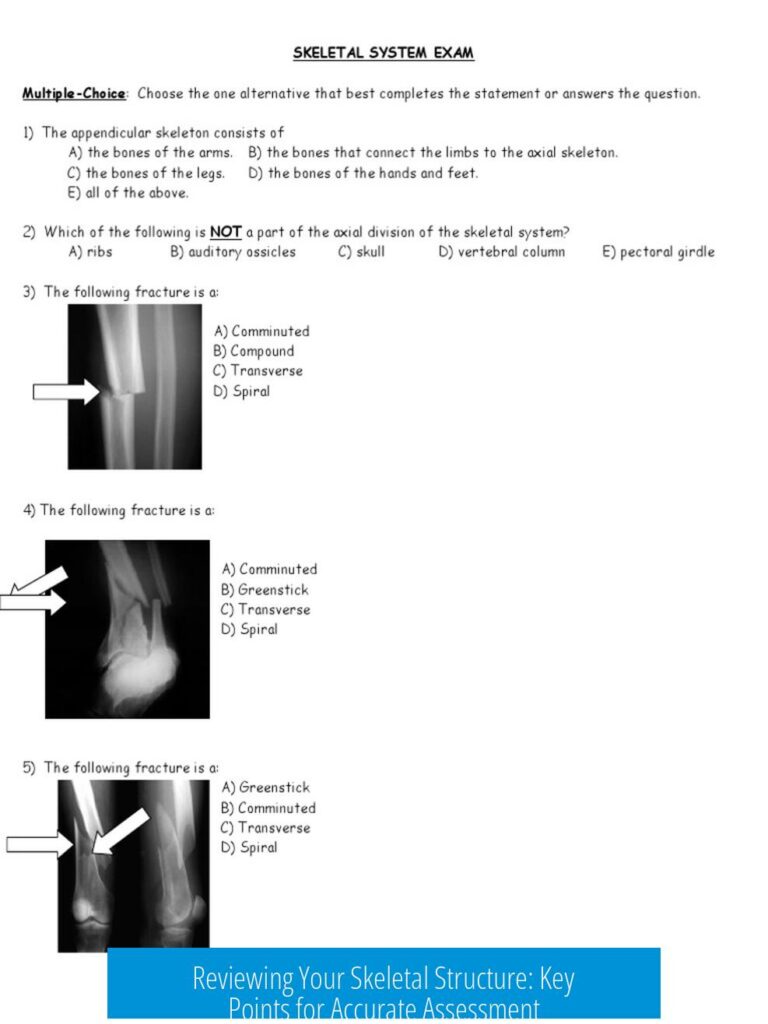
This color-coded periodic table derives from a creator known for contributing many of Wikipedia’s chemistry graphics, providing a practical and widely referenced palette. The source reflects community-driven efforts to unify the diverse coloring systems spreading across chemistry teaching and molecular modeling tools. The creator’s work, available here, offers a visually appealing and functionally informative chart useful in educational and research contexts.
Color Transitions and Visual Appeal
One highlight of this scheme is the smooth color transitions, notably within the lanthanoides group. This gradient reflects the periodic nature of elemental properties and enhances the overall look and understandability of the table.
- Gradients help users mentally group elements by chemical families.
- Color changes ease the recognition of element categories and trends.
Users remark that seeing the periodic table colored this way aligns with their internal mental models, making complex concepts more approachable on paper or screen.
Challenges with Specific Element Groups
Chalcogen Series

The chalcogen group reveals a particular challenge: selenium (Se) and tellurium (Te) appear with very similar colors. This similarity complicates distinguishing between them in digital or printed models, especially since their chemistry overlaps.
Some users adapt by recoloring tellurium with iodine’s distinctive purple hue. This adjustment improves visual distinction but reflects a workaround rather than a firm standard.
Transition Metals
Transition metals present significant difficulties due to their similar colors in the scheme. Chemists focusing on inorganic or mineralogical studies find this problematic because distinguishing metals like manganese (Mn), iron (Fe), and sodium (Na) is essential in complex compounds.
Despite this, the color scheme avoids placing similarly colored metals side by side in common compounds. This design consideration helps minimize confusion during molecular modeling.
Iron (Fe) Coloring Specifics
Iron in many fields is traditionally depicted in rust-like tones. Alternative colors, such as periwinkle, feel unnatural or odd to some professionals. This perception varies by subfield and individual experience. Likewise, ruthenium’s color changes with use—from purple to orange or brown—illustrate how color associations shift based on researcher exposure.
Color Consistency and Standardization Challenges
Overall, there is no universal color standard accepted worldwide for molecular models’ atom colors. Multiple kits and educational systems use differing schemes beyond the common black (carbon) and red (oxygen) conventions.
The value of a “color-coded periodic table” lies in providing a reference that avoids misidentifying elements in complex molecular structures. Especially in interdisciplinary work, a shared color guide reduces confusion across computational chemistry software and cross-disciplinary research communities.
Practical Considerations and Recommendations
- Color schemes should follow gradients within groups to maintain visual logic and ease identification.
- Colors like neon or helium’s, requiring fewer distinctions, still benefit from avoiding random color choices to preserve group coherence.
- The noble gases share colors with transition metals because they appear infrequently in molecular models, which is practical but leads to potential similarity confusion.
- Colorblind users face accessibility challenges with nuanced or similar hues in this scheme.
- Background colors’ meanings are unclear or underutilized in this approach, potentially limiting additional information delivery through color coding.
Variability by Educational and Regional Origins
Color assignments differ by institution or country. For instance, some schools assign oxygen light blue and hydrogen red, deviating from the black-red typical pairing. This diversity implies that complete standardization is unlikely and local adaptation persists.
Community Feedback and Observations
- Enthusiasm surrounds the chart’s ability to clarify element distinctions.
- Users question the absence of the heaviest elements like nihonium through oganesson, though these are less commonly modeled.
- Some wonder about the base rationale behind the chosen colors, noting it often reflects tradition and user consensus rather than formal codification.
Summary: Key Insights
- The periodic table with standard molecular model colors functions as a widely used but not formally standardized guide.
- Color gradients aid in identifying element groups but pose challenges in groups like chalcogens and transition metals.
- Variations exist by discipline, education, and software, making this scheme more a helpful convention than a strict norm.
- Accessibility concerns for colorblind individuals remain a caveat in the current palette design.
- The scheme’s origin from a respected Wikipedia contributor lends it practical credibility.
What is the source of the standard colors used in this periodic table?
The colors come from a Wikipedia user who created many chemistry graphics. They aimed to match typical molecular model colors for clarity and consistency.
Why are selenium and tellurium’s colors considered problematic?
They look too similar, making it hard to distinguish them in models. Some users recolor tellurium to iodine’s color for better contrast.
How does this color scheme handle transition metals?
Many transition metals have similar colors, which can be confusing. However, no two similarly colored elements are designed to coexist in the same compound for clarity.
Is there a universal standard for atom colors in molecular models?
No, different kits use different colors. This table provides a common reference to help reduce confusion across various tools and disciplines.
Are there accessibility concerns with this color scheme?
Yes, the color choices may not be ideal for colorblind users, which limits accessibility for some people using these molecular models.



Leave a Comment