What Actually Is Screening Effect and How Does It Work?
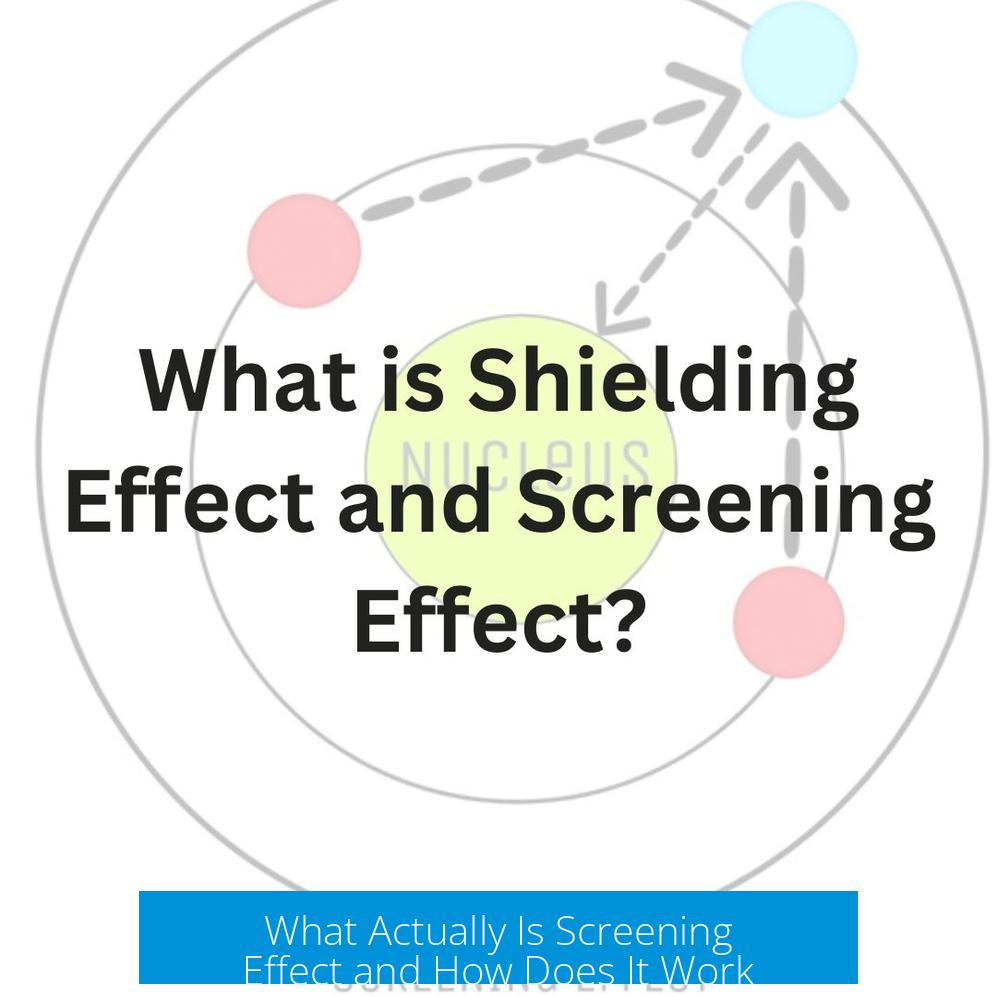
The screening effect refers to the phenomenon where inner electrons block or reduce the full positive charge of the nucleus felt by outer electrons in an atom. This effect alters the effective nuclear charge experienced by valence electrons, impacting atomic properties like size and ionization energy.
Understanding Screening: Core Electrons Block Nuclear Charge
Screening occurs because core electrons, which occupy orbitals closer to the nucleus, act like a barrier, reducing the positive charge that outer electrons experience. Instead of thinking of electrons repelling each other, it is more accurate to view core orbitals as physically obstructing the nucleus’s charge.
Core electrons spend most of their time near the nucleus. This proximity allows them to partially neutralize or “cancel out” the nuclear charge when viewed from the vantage point of an outer electron. In effect, an outer electron sees less than the full nuclear charge.
Distance from the Nucleus and Screening Efficiency
Screening effectiveness strongly depends on the distance between electrons and the nucleus. Electrons close to the nucleus better counterbalance the nuclear charge because their electron density overlaps significantly with the protons.
For example, consider a hydrogen atom at an extremely distant location compared to the nucleus-electron distance. At that far point, the positive charge of the nucleus and the negative charge of the 1s electron nearly cancel out, resulting in a net charge close to zero.
Screening Compared to Electron-Electron Repulsion
- It’s important to separate screening from electron-electron repulsion.
- Effective nuclear charge calculations focus only on the interaction between one electron and the nucleus, ignoring direct repulsions.
- Electron-electron repulsions are accounted for separately when solving Schrödinger’s equation in quantum mechanics.
Including electron repulsion within the screening effect would lead to double counting because repulsions are already part of the quantum mechanical treatment.
Summary of Screening Effect Key Points

- Screening arises from core electrons reducing the full nuclear charge seen by valence electrons.
- Electrons closer to the nucleus provide more effective screening.
- Screening is distinct from electron-electron repulsion; the latter is included in quantum calculations separately.
- Effective nuclear charge is the net positive charge experienced by an electron after accounting for screening.
What Actually Is Screening Effect and How Does It Work?
Understanding the screening effect can be tricky at first glance. Think of it as the inner electrons of an atom acting like cozy bodyguards for the positive nucleus, blocking some of its pull from the outer electrons. But how exactly does this blocking—this screening—work? Let’s unwrap this concept clearly and see why it matters in chemistry and physics.
In simple terms, the screening effect describes how inner-shell electrons reduce the effective nuclear charge felt by outer-shell electrons. They don’t push these outer electrons away. Instead, they physically stand between the nucleus and the outer electrons, partially masking the nucleus’ positive charge. Imagine a bunch of kids lined up: the front kids block the teacher’s view of the kids in the back. That’s classic screening in atomic terms.
The Core Idea: Core Orbitals Blocking Nuclear Charge
Picture the nucleus as a shiny magnet pulling electrons towards it. The inner electrons—those in core orbitals—are closer to the nucleus and act like screens. They don’t repel the outer electrons; rather, they sit in front of the nucleus, lessening the direct pull on the outer electrons. So, it’s best to think of screening as orbitals “blocking” the positive nuclear charge rather than repulsion between electrons.
This means electrons deep inside the atom create a shield. They reduce how much “magnetism” the nucleus exerts on those electrons farther out. The more inner electrons there are, the thicker this shield or screen becomes.
Distance Matters: Why Proximity to Nucleus Changes Screening
Imagine standing a mile away from a campfire. You might feel its warmth fade. Similarly, in an atom, if you pick a point really far from the nucleus—say centimeters away (gigantic in atomic terms)—the nucleus’s positive charge feels nearly neutral.
Why? Because in an atom like hydrogen, there’s one proton attracting one electron. Together, they cancel out their charges at large distances, making the entire atom electrically neutral. So, outer electrons farther from the nucleus experience less pull because core electrons close to the nucleus handle most of the charge.
Here’s a cool twist: the closer an inner electron is to the nucleus, the better it cancels out the positive charge from the nucleus. That’s why screening is more effective when electrons are closer in. Closer inner electrons mean a stronger screen for the outer ones.
The Inner Electron’s Job: Better At Screening Because of Position
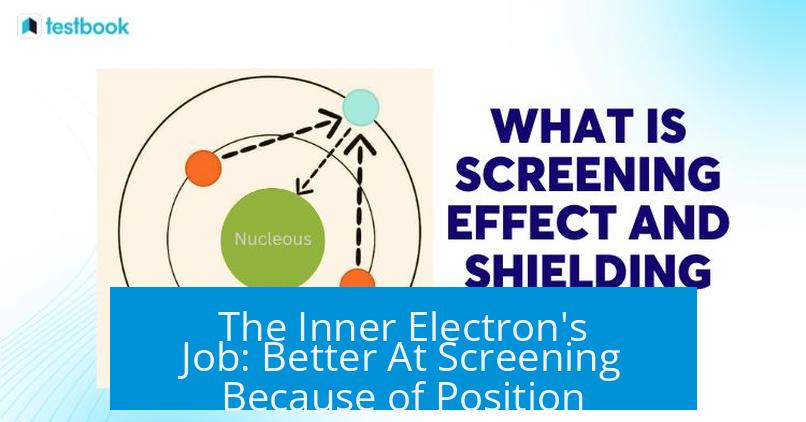
If we think of electrons as particles hanging around the nucleus, those closer to it spend most of their time near it. This proximity means the inner electrons can more thoroughly “cover” the nucleus from the view of outer electrons.
Interestingly, two outer electrons may be very far apart—literally on opposite ends of the atom! But an inner electron is always near the center, so it’s generally closer to any given outer electron than the outer electrons are to each other.
This explains why inner electrons dominate the screening effect: their position allows them to be effective shields, reducing the nuclear pull felt by electrons further out in the atom.
Separating Screening From Electron-Electron Repulsion
Now, here’s where many get tangled up: screening effect isn’t about electron-electron repulsion. These two concepts get confused a lot, but they serve different roles in atomic physics.
Electron-electron repulsion describes how electrons interact with each other, pushing apart due to their like charges. Screening, on the other hand, focuses solely on the interaction between the nucleus and a particular electron.
When scientists calculate atomic behavior, they use the “effective nuclear charge” to simplify. This effective charge considers only how much nuclear pull an electron actually feels after screening by inner electrons.
How Effective Nuclear Charge Fits Into Quantum Calculations
Effective nuclear charge isn’t just a theoretical notion—it’s a practical tool in quantum chemistry.
For example, when solving the Schrödinger equation, which governs electron behavior, chemists replace the true nuclear charge with this effective charge. This helps predict states and energies of electrons more accurately.
Why? Because the Schrödinger equation already accounts for electron-electron repulsions. So, if you factored those repulsions into effective nuclear charge as well, you’d double-count and mess up your results.
Bottom line: effective nuclear charge = nuclear attraction minus screening by inner electrons, ignoring electron-electron repulsion. The repulsion is handled separately in full quantum models.
A Quick Example: Sodium Atom
Take sodium, with 11 electrons. Ten of them fill inner shells, and one sits in the outer shell. The 10 inner electrons shield the outer one from much of the +11 nuclear charge.
Instead of feeling the full pull of +11, the outer electron feels an “effective nuclear charge” closer to +1. This dramatically affects sodium’s chemistry—it’s why sodium tends to lose that outer electron easily, becoming a positive ion.
Why Should You Care About Screening Effect?

- Chemical Reactivity: Screening affects how tightly outer electrons are held, influencing how atoms bond and react.
- Atomic Size: The stronger the screening, the smaller and less tightly bound outer electrons are, making atoms larger or smaller.
- Periodic Trends: Understanding screening clarifies why elements down a group behave similarly and how effective nuclear charge trends explain atom properties.
So, next time you look at the periodic table or wonder why potassium reacts like it does, remember the tiny shield of inner electrons—the unsung heroes that screen the nuclear charge and shape the entire behavior of elements.
Wrapping It Up: The Screening Effect, Demystified
Here’s the crystal-clear truth: screening effect is the inner electrons physically blocking the nucleus’s positive charge, reducing the pull felt by outer electrons. It’s not electron repulsion; it’s more like a shield or curtain.
Distance plays a starring role. Inner electrons near the nucleus screen charge better than those far away. The concept of effective nuclear charge emerges from this, simplifying complex atomic interactions by focusing on just the nucleus-to-electron attraction after accounting for screening.
Finally, remember screening and electron-electron repulsion are distinct forces in an atom. Screening adjusts the nuclear pull, while repulsion deals with how electrons push each other around. Clever physicists keep these separate to get the math just right.
Feeling screened yet? Hopefully, this spotlight on the screening effect pulled back the curtain for you on one of atomic theory’s key players.
What is the screening effect in atoms?
Screening occurs when inner electrons block the positive charge of the nucleus. These core electrons reduce the attractive force felt by outer electrons. The screening effect lowers the net nuclear charge experienced by valence electrons.
How does distance from the nucleus affect screening?
Electrons closer to the nucleus screen more effectively. Inner electrons are better at canceling nuclear charge because they spend more time near the nucleus. Outer electrons feel reduced charge due to this blockage.
Does electron-electron repulsion contribute to the screening effect?
Electron-electron repulsion is separate from screening and effective nuclear charge. Screening only considers how inner electrons block nuclear charge. Repulsion effects are handled separately in quantum calculations.
Why can’t electron-electron repulsion be included in effective nuclear charge?
Including electron-electron repulsion in effective nuclear charge would double count these interactions. Effective nuclear charge only accounts for nucleus-to-electron attraction, not repulsions between electrons.
How is effective nuclear charge used in quantum mechanics?
Effective nuclear charge replaces the actual nuclear charge in the Schrödinger equation. This approach simplifies calculations by incorporating screening effects while treating electron repulsions separately in the equation.



Leave a Comment