What Does it Mean When a Bond Is More Polar?
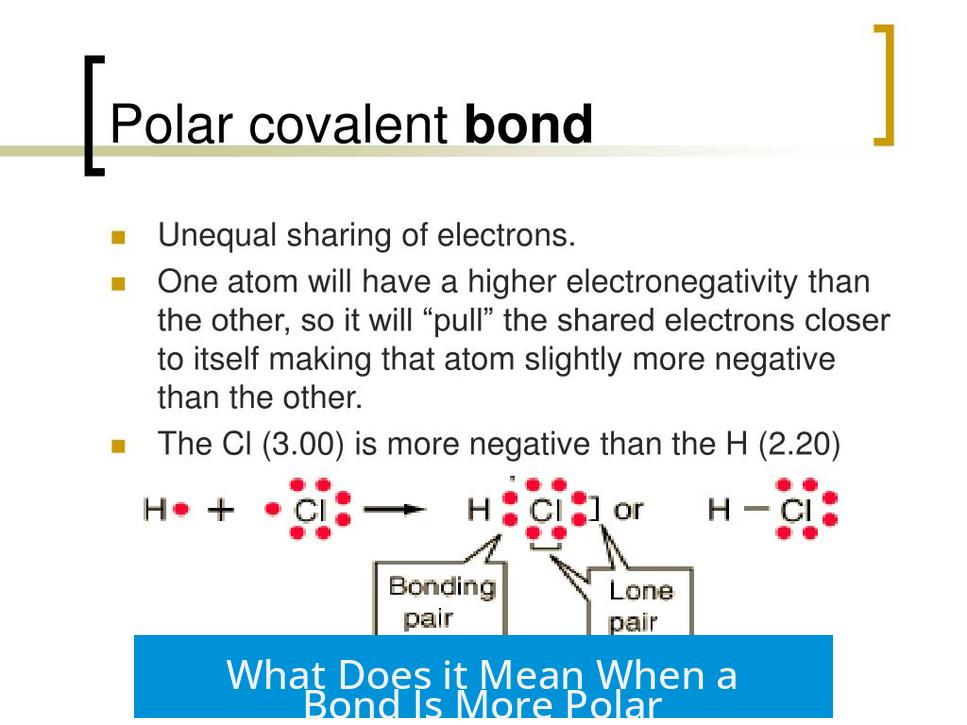 A bond is more polar when electron density shifts significantly toward the more electronegative atom, creating greater charge separation and a stronger dipole moment. This uneven distribution of electrons changes the bond’s characteristics and influences molecular interactions.
A bond is more polar when electron density shifts significantly toward the more electronegative atom, creating greater charge separation and a stronger dipole moment. This uneven distribution of electrons changes the bond’s characteristics and influences molecular interactions.
Electron Density and Electronegativity

In a chemical bond, electrons are shared between atoms. When one atom has higher electronegativity, it attracts electrons more strongly. This shifts electron density toward that atom. The bond’s strength often increases as the electron cloud becomes more localized around the more electronegative atom.
Charge Separation and Dipole Moment
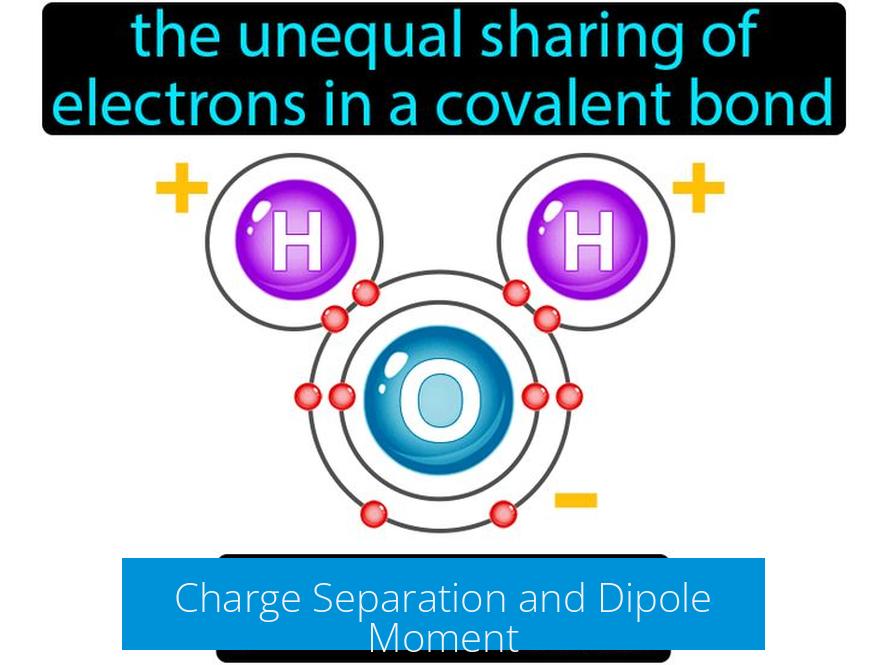
Polarity relates directly to charge separation within a bond. Greater difference in electronegativity means more uneven electron sharing, resulting in partial positive and negative charges on the atoms. This creates a dipole moment. A highly polar bond has a larger dipole moment, indicating significant charge separation. Bonds with smaller differences have less polarity and weaker dipole moments.
Measuring Bond Polarity
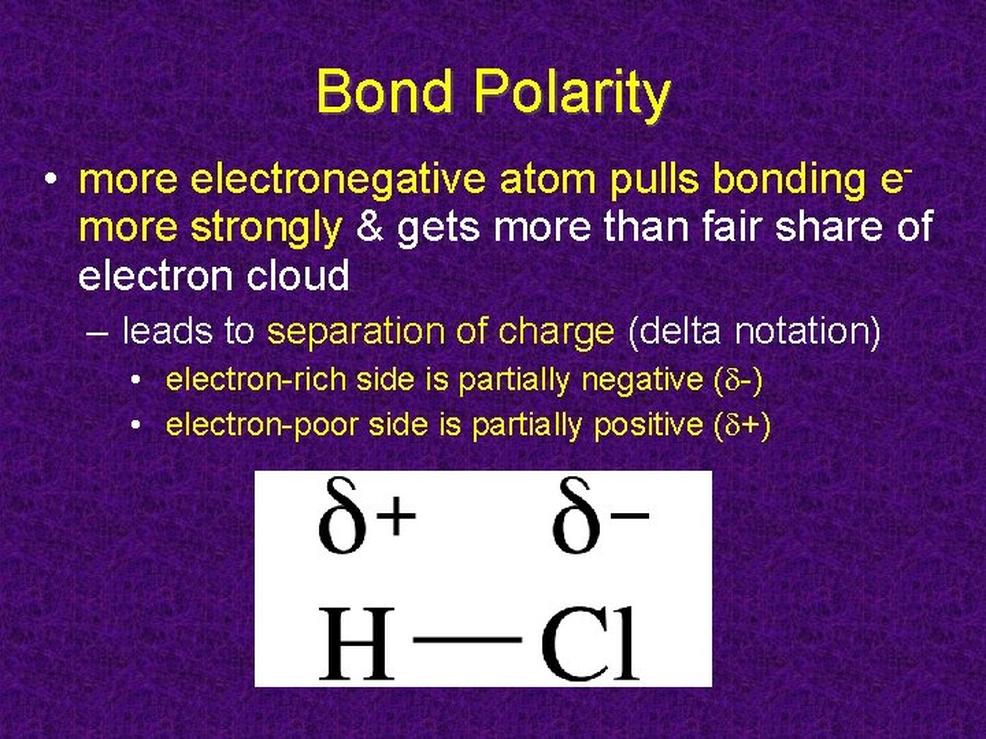
Bond polarity is quantified by the difference in electronegativity values between the two atoms involved. Subtracting the smaller electronegativity from the larger one yields this difference. A larger difference corresponds to a more polar bond. For example, carbon-fluorine (C-F) bonds are more polar than carbon-chlorine (C-Cl) bonds because fluorine has a higher electronegativity than chlorine.
Effects of Bond Polarity
Polar bonds influence physical and chemical properties. They affect solubility and interactions with solvents. Polar bonds can enhance a molecule’s ability to dissolve in polar solvents and affect its behavior in processes like chromatography. Additionally, polarity impacts molecular geometry and reactivity.
Comparative Nature of Polarity
Polarity is always relative. Saying a bond is “more polar” requires comparing it to another bond’s polarity. For instance, C-F is more polar than C-Cl by comparing their electronegativity differences, providing a reference for understanding bond behavior.
Key Takeaways
- More polar bonds have larger electronegativity differences between atoms.
- Electron density shifts toward the more electronegative atom, increasing bond polarity.
- Polarity causes charge separation and increases the dipole moment.
- Bond polarity affects solubility, reactivity, and physical properties.
- Polarity is comparative and depends on reference bonds for context.
What Does it Mean When a Bond is More Polar?
When a bond is more polar, it means there is a greater unevenness in the way electrons are shared between two atoms. The electrons tend to stick closer to one atom, making it partially negative, while leaving the other atom partially positive. This separation of charge is what defines polarity.
Now, that might sound straightforward, but there’s a surprising amount packed into the simple idea of “more polar.” Let’s unpack this mystery together.
Electron Density and Electronegativity: The Tug of Electrons
Imagine two atoms holding an electron rope. If one atom is stronger at tugging—thanks to greater electronegativity—it pulls the electrons closer to itself. This “tug” creates an unbalanced electron cloud.
Electron density shifts toward the more electronegative atom, which means the atoms don’t share electrons equally anymore. Picture a crowd leaning toward one side of a seesaw. This shift is what makes the bond more polar. The bigger the tug, the bigger the shift.
Here’s a fun fact: electronegativity is like the atom’s charm for electrons. Fluorine is the “celebrity” with a very high electronegativity, always drawing electrons into its camp. Carbon’s electronegativity is much lower, so bonds between carbon and fluorine end up with strongly polar character. More on that later.
Charge Separation and Dipole Moment: The “Electric” Personality of Bonds
What does this electron shift mean exactly? It means the bond gets a dipole moment, which is basically the separation between positive and negative charges in the bond. Think of it as a tiny battery with a positive side and a negative side.
More polar bonds have a higher dipole moment. That means the partial charges are more distinct. Less polar bonds have these charges smeared more evenly, resulting in a lower dipole moment.
If you’ve ever played with magnets, think of poles. The stronger and more separated they are, the more they can pull or push other magnets around. More polar bonds behave like stronger magnets within molecules, impacting how the molecules interact.
Effects of Bond Polarity: Why Do We Even Care?
Okay, so a bond’s polarity is about electron tug-of-war and tiny electrical charges. But why should anyone outside chemistry nerd territory care?
Polarity influences everything from how molecules stick together, to what solvents they mix well in, to even the products we taste and smell. In the world of chemistry and biochemistry, polarity decides if a molecule dissolves in water or oil, how it moves in chromatography, and what kind of reactions it can undergo.
For example, in chromatography (think of it as a molecule race using different tracks), molecules with more polar bonds often stick better to certain phases. This means if you’re trying to separate chemicals, knowing polarity helps you predict follow-the-leader behavior.
Measurement of Bond Polarity: Electronegativity Difference as the Scorecard
How do scientists quantify how “polar” a bond is? They subtract the electronegativity of one atom from the other. The bigger the difference, the more polar the bond.
Here’s a quick math moment: if atom A has an electronegativity of 3.5 and atom B has 2.0, then 3.5 – 2.0 = 1.5. That’s a good-sized gap indicating a fairly polar bond.
This electronegativity difference doesn’t just tell us about polarity; it hints at the bond strength and character. Huge differences may even suggest ionic bonds, where electrons leap all the way to one atom rather than sharing.
Comparative Nature of Polarity: Always Compare to Something Else
Polarity is like spiciness in food: it only means something when compared.
Take the example of C-F versus C-Cl bonds. Carbon-fluorine bonds are more polar than carbon-chlorine bonds. Why? Because fluorine is more electronegative than chlorine, pulling the electron density harder. But it’s essential to have this relative scale; “more polar” needs a reference.
This is a reminder: chemistry loves comparisons. Saying a bond is “more polar” always means it is more polar than something else.
Putting It All Together: What Does a More Polar Bond Really Do?
When you zoom out, the meaning of a “more polar bond” is crystal clear and actually super practical:
- It tells us where electrons prefer to hang out.
- It reveals how the molecule behaves electrically—its dipole moment.
- It predicts interactions between molecules and with solvents.
- It guides scientists on how molecules separate, react, and fit together.
- And it provides a measurable scale via electronegativity differences.
One practical takeaway? In your chemistry experiments or studies, spotting a more polar bond means expecting stronger charge separation. This predicts everything from solubility to reactivity. Should your molecule love water or hate it? Polarity may hold the answer.
Final Thoughts: Why The Fascination With Polar Bonds?
Polarity is a fundamental concept hiding in plain sight. It underpins molecular behavior without the flashy drama of explosions or fireworks. Yet, without understanding polarity, you miss half the picture of how molecules talk, bond, and break apart.
Thinking about bonds in terms of polarity opens doors to smarter product design (including drugs and materials), better lab work, and a deeper grasp of nature’s microscopic world. So, the next time someone asks you, “What does it mean when a bond is more polar?”—you’ll have a solid answer and maybe even a smile ready.
Got a favorite example of polar bonds changing everything? Share it! Chemistry is more fun with stories.




Leave a Comment