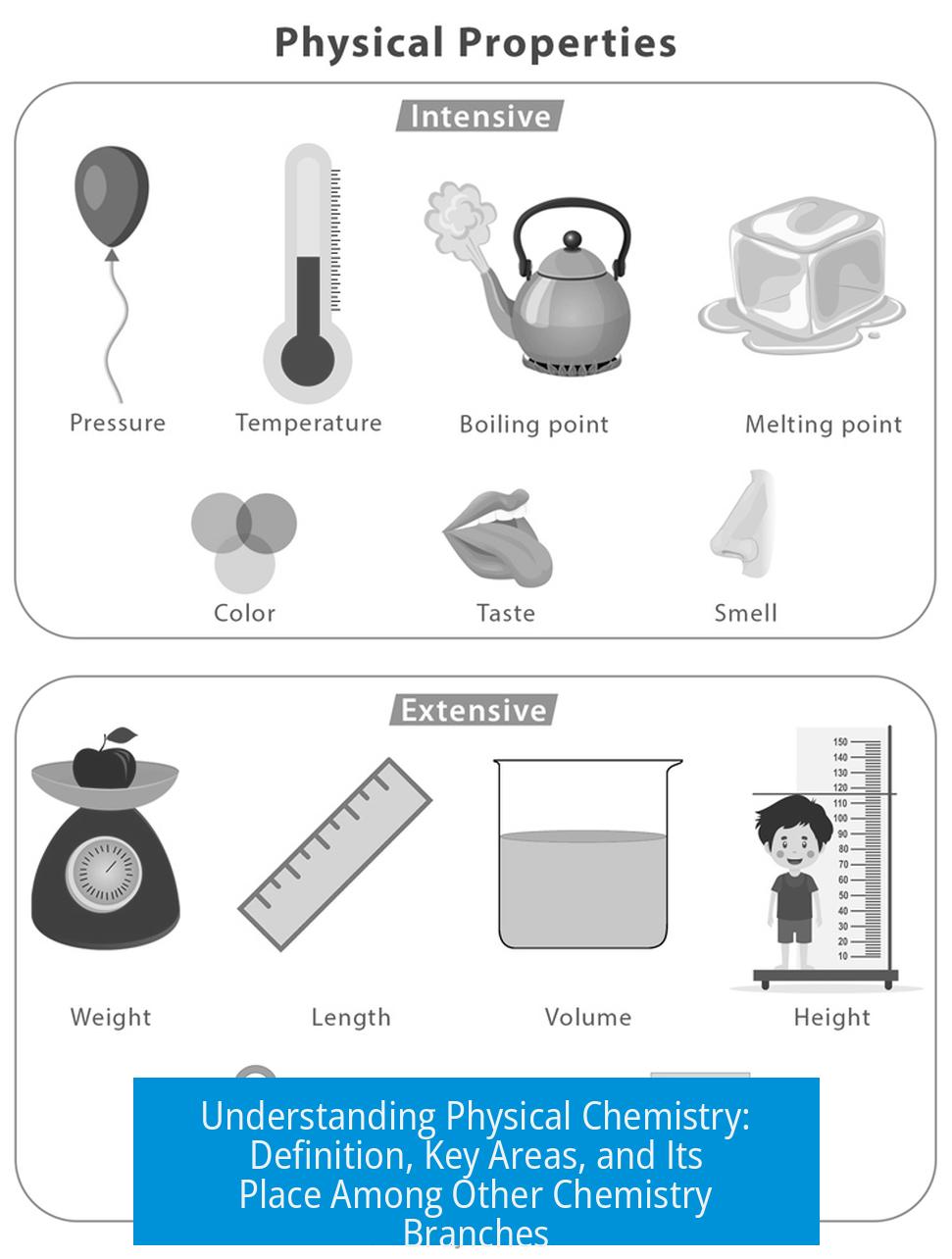
What is Physical Chemistry?

Physical chemistry applies principles of physics and mathematics to understand chemical phenomena. It uses mathematical models and theories to explain how chemicals behave on atomic and molecular levels.
Definition and Nature
Physical chemistry bridges physics and chemistry. It relies heavily on mathematics, similar to physics courses where math describes natural processes. This branch examines matter in chemical reactions, focusing on energy, structure, and rates of reactions through quantitative methods.
Key Areas within Physical Chemistry
- Quantum Mechanics: Studies atomic and molecular systems using quantum laws, explaining chemical bonding and electronic structures.
- Thermodynamics: Focuses on energy changes in reactions and phase transitions, analyzing heat, work, and spontaneity.
Physical Chemistry Versus Other Chemistry Branches
Physical chemistry is often seen as rigorous and precise. For instance, Sinclair Lewis’s Arrowsmith compares it to organic chemistry, emphasizing its exactness and fundamental role in understanding chemistry deeply.
Public Perception and Humor
Some view physical chemistry humorously, seeing it as applying simple math equations like y=mx+b to complex chemical problems. Despite this, its mathematical rigor is vital for scientific accuracy.
Educational Resources
Courses like the Physical Chemistry MOOC offer structured syllabi covering quantum mechanics, thermodynamics, and kinetics. These resources help students grasp the sophisticated concepts physical chemistry entails.
Summary
- Physical chemistry uses physics and math to explain chemical behavior.
- Key fields include quantum mechanics and thermodynamics.
- It provides precise, quantitative models for chemical phenomena.
- Recognized for rigor and foundational importance in chemistry.
- Educational materials like MOOCs are available for deeper study.



Leave a Comment