Difference Between Carbonyl Group and Acyl Group
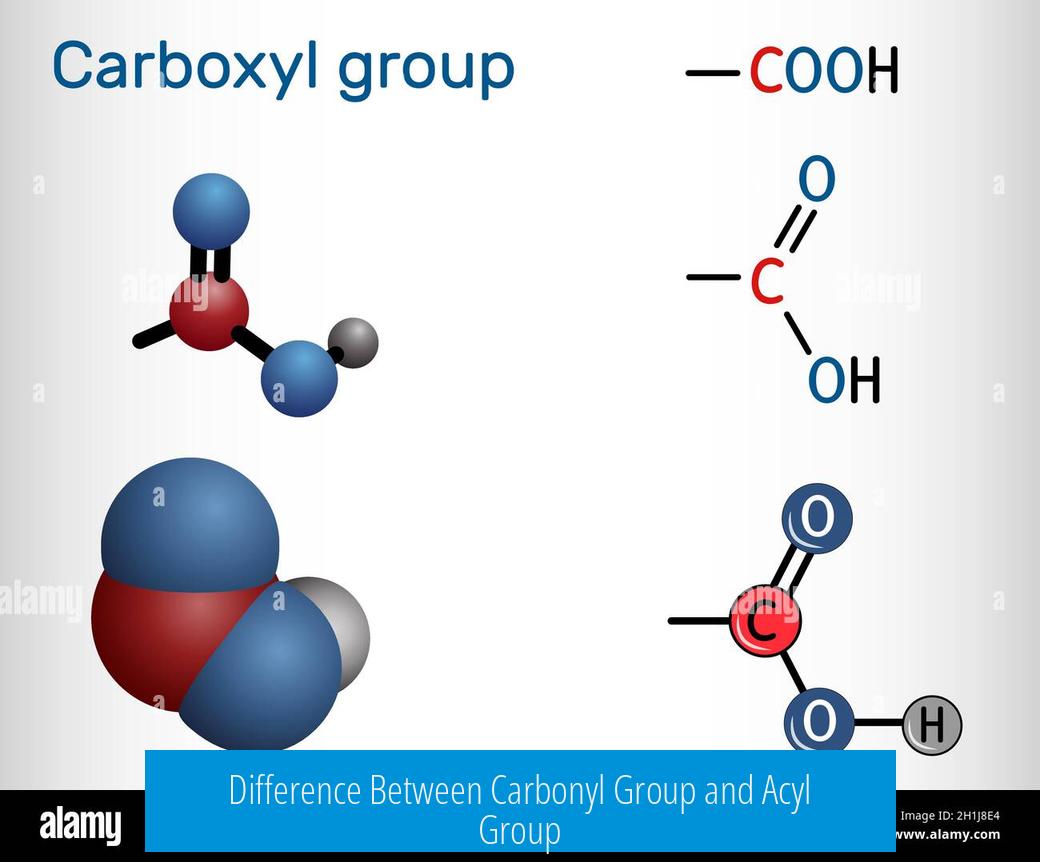
A carbonyl group is a broad functional group characterized by a carbon atom double bonded to oxygen (C=O), found in various organic compounds. An acyl group is a specific type of carbonyl group where the carbonyl carbon is bonded directly to an alkyl or aryl group (–C(=O)R), often derived from a carboxylic acid.
What Is a Carbonyl Group?
The carbonyl group is a general term for any molecular fragment containing a carbon-oxygen double bond. This includes functional groups like ketones, aldehydes, esters, amides, and carboxylic acids. Its defining feature is the C=O bond, but the surrounding atoms or groups attached to the carbon vary widely.
Because carbonyl is a generic term, it does not specify the chemical behavior or the identity of adjacent groups. It is used neutrally to describe the presence of the double bond in different organic molecules.
What Is an Acyl Group?
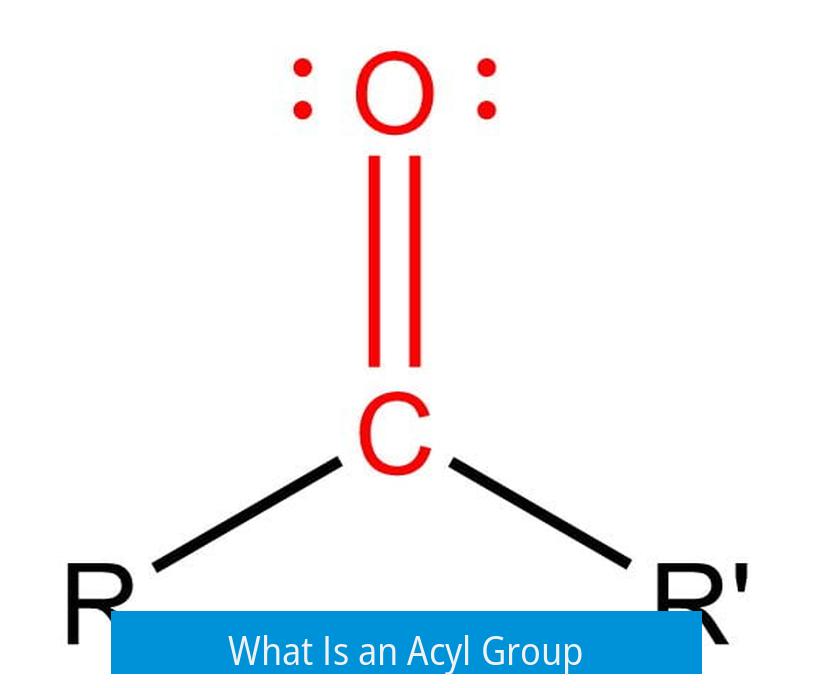
An acyl group refers specifically to a carbonyl carbon bonded directly to an alkyl or aryl moiety. It is often derived from carboxylic acids by removing the hydroxyl group (–OH). The acyl group formula is generally represented as –C(=O)R, where R is an alkyl or aryl substituent.
Acyl groups commonly appear in contexts involving chemical reactions where they are transferred or added to other molecules. For example, in Friedel-Crafts acylation, an acyl group attaches to an aromatic ring through electrophilic substitution.
Context and Usage Differences
- Carbonyl: Used broadly to indicate any compound with C=O. It lacks directional or reaction-specific implications.
- Acyl: Used when the carbonyl group is part of a reactive substructure, often in synthetic routes or functional group transformations.
The acyl group implies a more specific chemical environment, typically linked to an alkyl or aryl group and often involved in forming new chemical bonds. The carbonyl term serves as a neutral descriptor without implying reactivity or substitution patterns.
Summary Table
| Aspect | Carbonyl Group | Acyl Group |
|---|---|---|
| Definition | Carbon double bonded to oxygen (C=O) in any compound | Carbonyl carbon bonded to alkyl or aryl group (–C=O-R) |
| Scope | General functional group including ketones, aldehydes, esters, etc. | Substructure derived from carboxylic acids, reactive subunit |
| Usage | Descriptive term without functional specificity | Functional term in chemical reactions, especially acylation |
| Chemical Role | Neutral, structural descriptor | Reactive group for bond formation |
Key Takeaways
- Carbonyl groups encompass any C=O bond in various molecules, lacking chemical context.
- Acyl groups are specific carbonyl-containing fragments attached to alkyl or aryl groups.
- Acyl groups often participate in reactions, such as acylation and bond formation.
- Carbonyl is a general structural term; acyl implies a reactive subunit derived from carboxylic acids.
What defines a carbonyl group compared to an acyl group?
A carbonyl group consists of a carbon double bonded to oxygen (C=O). It is a broad group found in many compounds. An acyl group is a specific carbonyl attached to an alkyl or aryl group, derived from carboxylic acids.
Do carbonyl and acyl groups represent full functional groups?
No, both terms describe substructures, not complete functional groups. They identify parts of molecules but do not define the whole chemical class or structure.
When is the term “acyl group” typically used?
The acyl group term is used when discussing adding or attaching the carbonyl plus alkyl/aryl part to another molecule. For example, in Friedel-Crafts acylation, the acyl group forms new bonds with aromatic rings.
Is the carbonyl group term linked to specific chemical reactions?
Carbonyl is a neutral term referring simply to the C=O motif. It does not imply any reaction role or the nature of groups attached to it. Its use is broad and non-specific.
Can an acyl group come from an aldehyde?
No, an acyl group is derived from carboxylic acids and their derivatives, not aldehydes. Aldehydes have a carbonyl but do not form acyl groups because they lack the proper alkyl or aryl attachment.




Leave a Comment