What State of Matter Is a Flame?
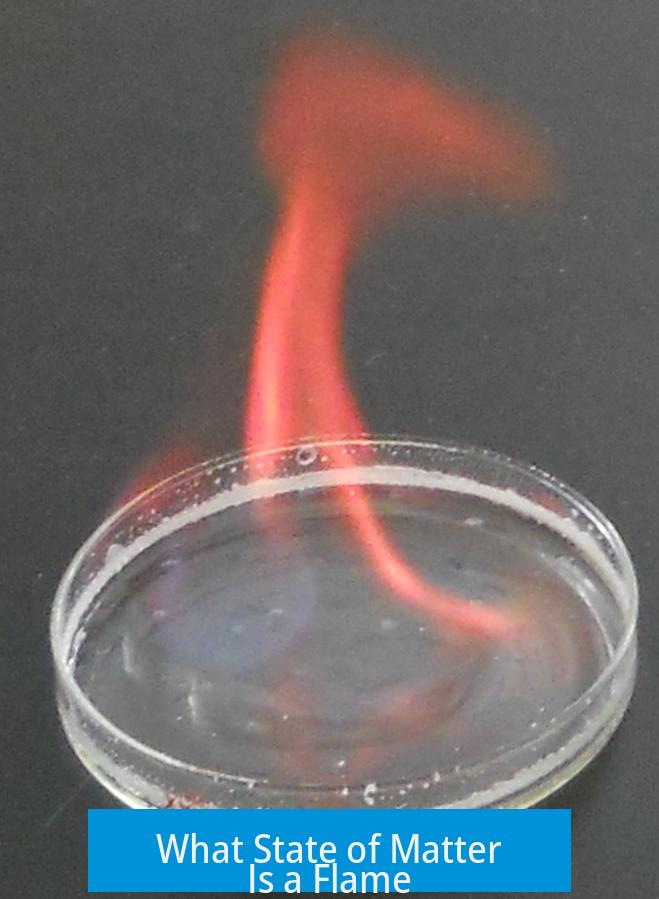
A flame primarily exists as hot gas that undergoes combustion, with parts turning into a weak plasma state due to partial ionization; it also contains solid particulates and vaporized liquids, making it a complex event involving multiple states of matter rather than a single clear-cut phase.
Flame as Hot Gas
At its core, a flame is composed of gases produced during combustion. These arise from the vaporization and chemical breakdown of fuel materials. Common gases in flames include carbon dioxide, carbon monoxide, methane, and water vapor. While some of these substances are liquids or solids at room temperature, the intense heat causes them to exist in the gaseous phase.
The flame’s visible region is localized to areas where chemical reactions occur rapidly. This confines the reaction zone but does not preclude the flame from being gaseous. It emits heat and light due to the combustion of these hot gases.
Flame as Weak Plasma
A flame exhibits plasma characteristics, which is often referred to as the fourth state of matter. Plasma contains ionized particles—electrons and ions—that result from high-energy conditions breaking atomic bonds. In flames, the temperature sometimes suffices to ionize gases partially. This ionization leads to charged particles existing transiently within the flame.
- The plasma present in flames is typically weak; the ion concentration is low.
- Flames are not fully ionized plasma like those seen in extreme environments such as stars or fusion reactors.
- Highly energetic flames, such as those in rocket engines, show stronger plasma characteristics and can generate magnetic fields.
This weak plasma state explains part of a flame’s luminous nature, but it is not the sole cause.
Origins of Flame Color and Light
The visible color of a flame, such as the yellow hue of a candle, arises mostly from incandescent solid particles, not from plasma radiation itself. These particles, typically soot and unburnt fuel fragments, heat until they glow, similar to the filament of an incandescent bulb.
A few points about flame coloration:
- Yellow and orange shades come from glowing soot particles and blackbody radiation.
- Bright species like sodium atoms can add to the orange flame color in campfires.
- Blue flames, often seen with alcohol, result from more complete combustion with less soot and thus less visible incandescent particles.
Thus, flame light involves multiple mechanisms, combining hot gas emission, particle incandescence, and some ionization effects.
Flame as a Mixture of Matter States
A flame is not simply one state of matter but a mixture:
| State of Matter | Components in Flame |
|---|---|
| Gas | Combustion gases like CO2, CO, CH4, and vaporized liquids |
| Liquid (in vapor form) | Fuel vapors evaporated due to heat |
| Solid | Glowing soot, ash particles, carbon remnants |
| Plasma | Partially ionized gas with free electrons and ions |
These constituents interact dynamically in a flame, making it an event where matter transforms and releases energy continuously.
Flame: Energy Emission from Matter
The flame itself is not a discrete substance or state of matter, but an energy emission. It results from chemical reactions where fuel molecules combine with oxygen, breaking bonds and re-forming into new products, while releasing heat and light.
In scientific and philosophical terms, fire is often considered a process or phenomenon representing energy transfer, rather than a matter form in isolation.
Key Takeaways
- Flames are primarily hot gases formed from fuel combustion, with vaporized liquids due to high temperature.
- Partial ionization within flames creates a weak plasma state, but flames lack full plasma properties seen in hotter environments.
- Visible flame colors mostly arise from incandescent solid particles like soot, not solely from plasma glow.
- A flame encompasses multiple states of matter simultaneously: gas, plasma, solid particles, and vaporized liquids.
- The flame is an energy-emitting event, not a standalone state of matter or substance.
What state of matter primarily describes a flame?
A flame is mainly a gas. It consists of hot gases from combustion, such as carbon dioxide and carbon monoxide. These gases are heated and in the gas phase during burning.
Is a flame considered plasma, and why?
Yes, a flame can be a weak form of plasma. The heat ionizes some gas molecules, creating charged particles like ions and electrons. However, candle flames have low ionization compared to hotter flames like those in engines.
Does the color of a flame come from plasma glow?
No. The yellow or orange color comes mainly from glowing solid particles like soot. Heat excites electrons in these particles, causing visible light. The ionized gas does not produce much of this color.
Do flames contain more than one state of matter?
Yes. Flames include gases, solid particles like soot, and a small amount of plasma. Liquids that evaporate at flame temperature can also be present in a gaseous phase. Flames are an event involving multiple states.
How does flame temperature affect its plasma state?
Higher flame temperatures increase ionization, causing more plasma formation. Very hot flames behave more like true plasma and can generate weak magnetic fields, unlike cooler flames.





Leave a Comment