Simple Ways to Differentiate Physical and Chemical Properties and Changes
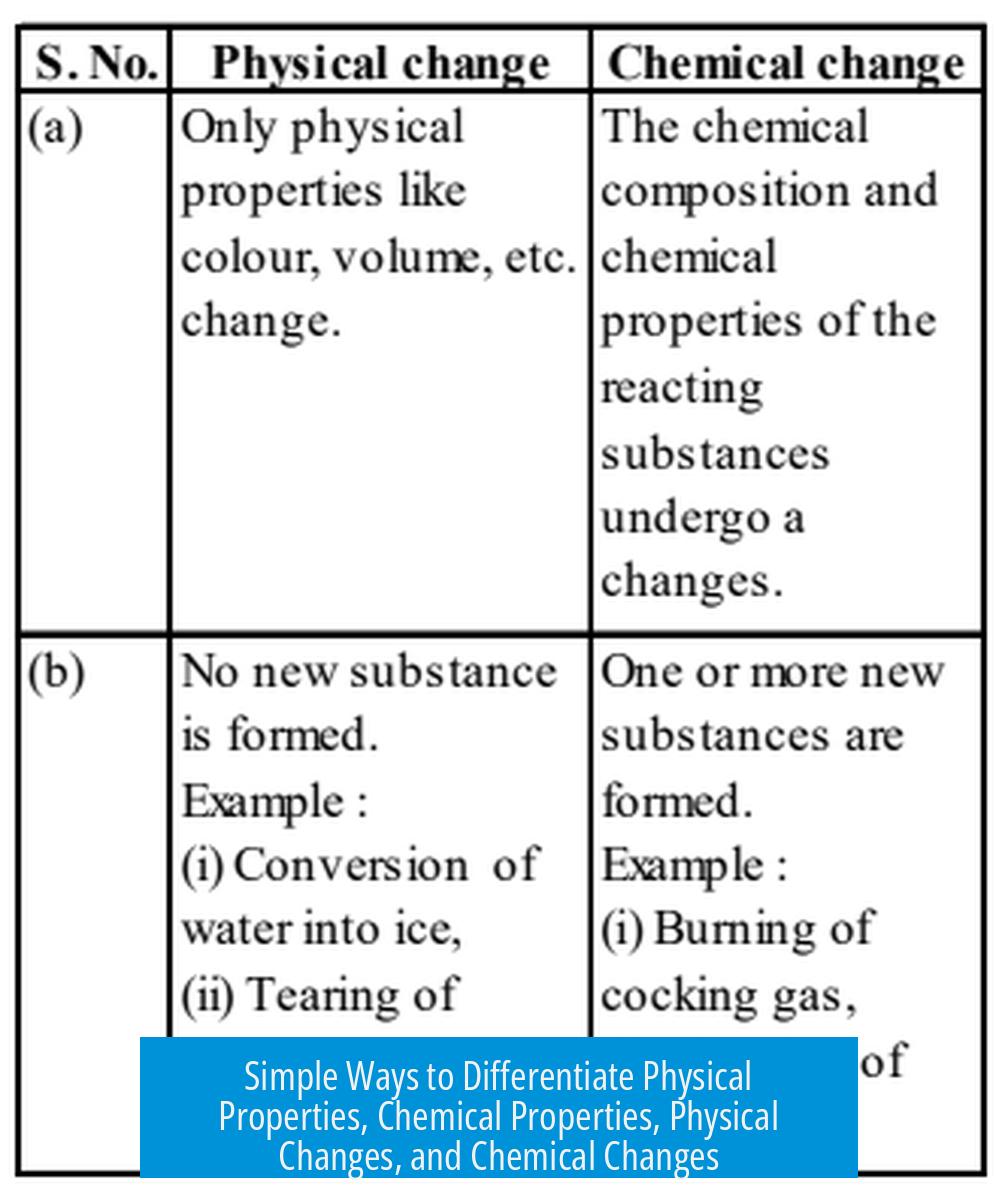
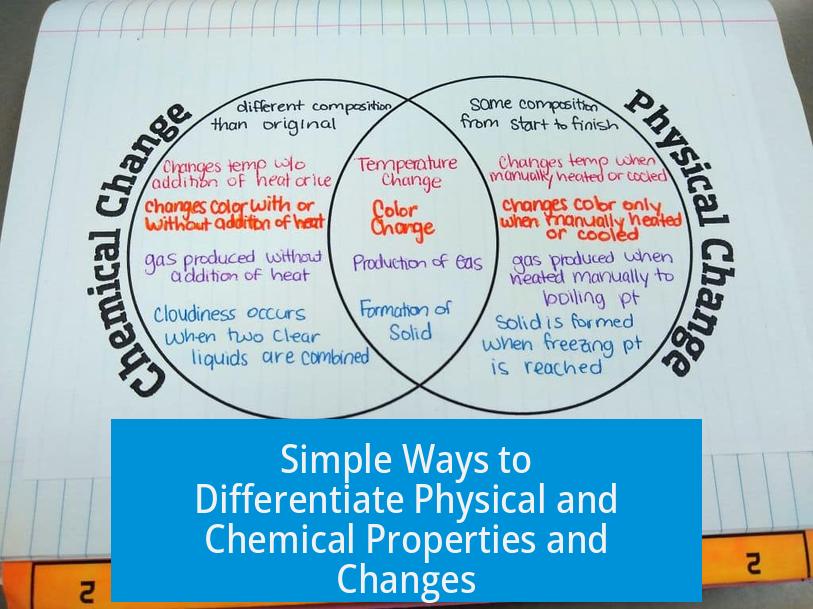 A physical property can be observed without altering the substance, while a chemical property requires changing the substance to a new one. A physical change alters the form but not the substance’s identity, whereas a chemical change transforms it into a different substance.
A physical property can be observed without altering the substance, while a chemical property requires changing the substance to a new one. A physical change alters the form but not the substance’s identity, whereas a chemical change transforms it into a different substance.
Physical Property
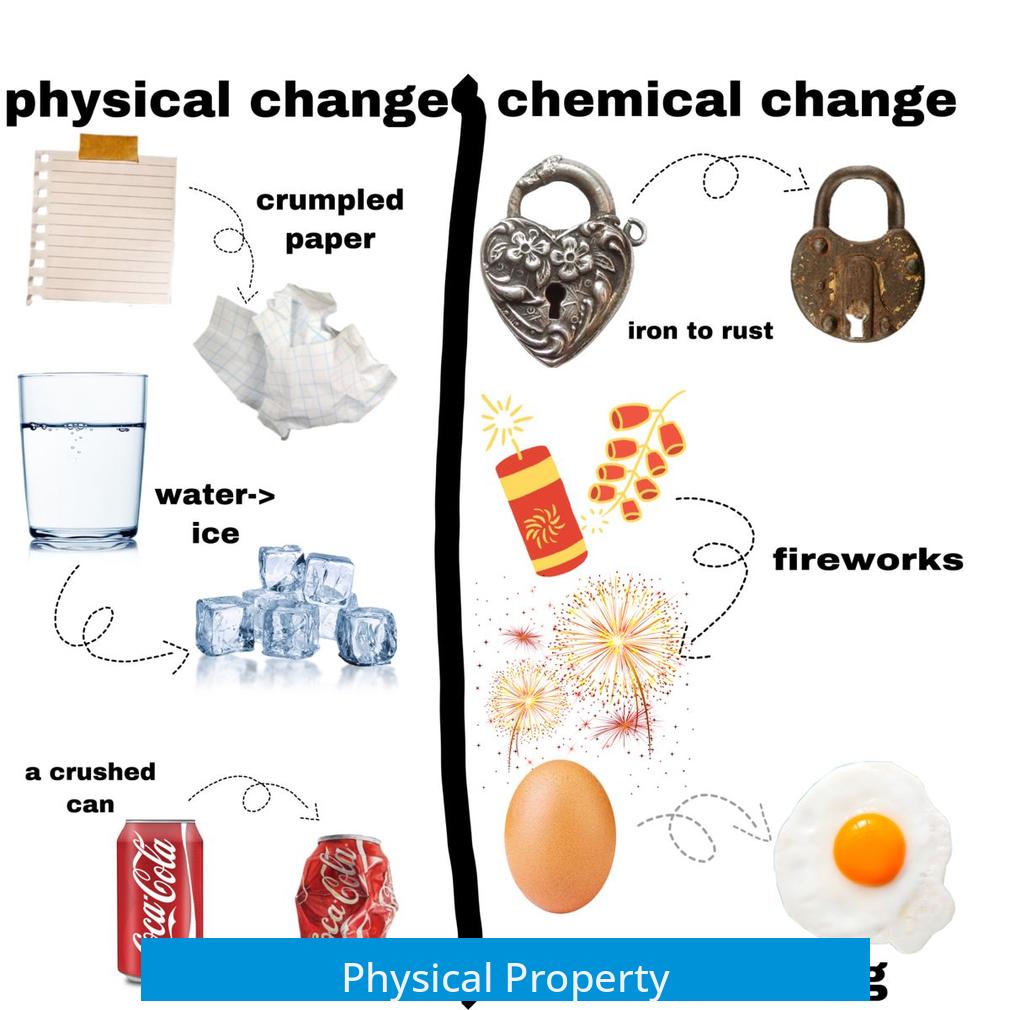
Physical properties describe characteristics observable without changing the chemical nature of a substance.
- Mass
- Density
- Boiling and melting points
- Color and texture
- Phase (solid, liquid, gas)
- Conductivity
These traits help identify or describe a material but do not involve chemical transformation.
Chemical Property

Chemical properties reveal how a substance reacts and can only be observed by forming new substances.
- Combustibility
- Flammability
- Acidity or alkalinity
- Reactivity with other chemicals
- Radioactivity
Observing these properties involves chemical reactions changing the original material.
Physical Change

This change affects the substance’s appearance or form without altering its chemical composition.
- Melting or freezing
- Boiling or condensation
- Cutting or crushing
- Phase transitions such as sublimation
For instance, melting sugar changes its phase but keeps its chemical structure intact.
Chemical Change
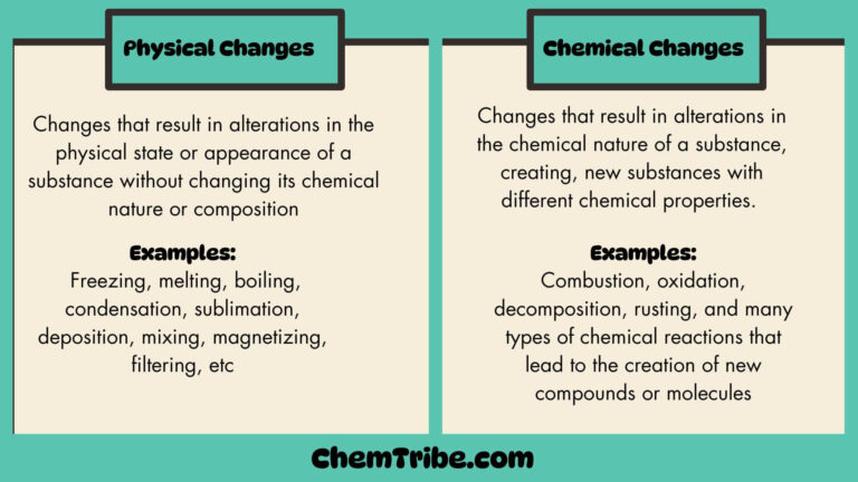
Chemical changes create new substances with different properties.
- Burning wood produces ash and gases
- Mixing baking soda and vinegar releases carbon dioxide
- Cooking eggs changes proteins chemically
- Dyeing hair rearranges molecular bonds in hair
Evidence includes gas release, color change, odor changes, temperature shifts, or light emission not caused by external heating or cooling.
Practical Illustrations
| Example | Physical Property | Chemical Property | Physical Change | Chemical Change |
|---|---|---|---|---|
| Hair | Color of hair | How easily it burns | Cutting hair | Dyeing or perming hair |
| Sugar | Sweet taste, crystalline solid | Flammability | Melting sugar | Burning sugar or caramelizing |
Key Points
- Physical properties are observed without substance change.
- Chemical properties require transforming the substance.
- Physical changes alter form or phase but keep identity.
- Chemical changes create new substances with new properties.
- Visual clues like color, odor, or temperature shifts help identify chemical changes.
What’s a Simple Way to Differentiate Physical Property, Chemical Property, Physical Change, and Chemical Change?
Simply put, a physical property is something you can observe without changing the substance itself, while a chemical property only reveals itself when the substance transforms into something new. Similarly, a physical change alters the form but not the substance, whereas a chemical change creates a new substance altogether.
Sounds straightforward? Let’s dive deeper and untangle this puzzle with some colorful examples and clear facts.
First, Meet the Properties: Physical vs. Chemical
A physical property describes a trait you can see or measure without turning the substance into something else. Think of the color of your hair (that’s a physical property), or how dense a rock feels in your hand. You don’t need to burn, melt, or change these things to observe these properties.
- Examples: mass, density, boiling point, color, texture, odor, conductivity, brittleness.
Now, a chemical property only reveals itself if the substance undergoes a chemical transformation. You can’t just look at it and know. You have to set it on fire, react it with acid, or otherwise change it to detect this property.
- Examples: combustibility (can it burn?), flammability, acidity or alkalinity, reactivity, radioactivity.
Physical vs. Chemical Change: The Identity Crisis of Substances
Ever wondered how to know if something’s undergone a physical change or a chemical change? Here’s the secret: Is the substance fundamentally the same or completely new?
A physical change means the substance changes shape, size, or state but keeps the same chemical identity. You could chop wood into smaller pieces and still have wood. You melt ice into water and water is still water.
- Examples: phase changes like melting, boiling, freezing, sublimation; cutting, crushing, molding.
Conversely, a chemical change means the substance becomes a totally new substance. You can tell because of signs like color shifts, smell changes, bubbles (gas), or temperature changes that occur without heating or cooling devices. Think of burning wood, baking a cake, or mixing vinegar with baking soda to make foam.
- Evidence: color or odor changes, gas production, temperature change, light or sound produced.
A Tale of Hair: Your Personal Chemistry Lesson
Let’s make it personal. Imagine your hair. The color you see every day is a physical property. It’s right there, plain to observe, no fire required.
If you trim your hair, that’s a physical change. The strands get shorter, but your hair’s chemical makeup remains untouched. No new substances are born!
Now, consider burning hair. How easily it burns? That is a chemical property. You can’t know that just by looking—it shows up when hair turns to ash.
And finally, when you dye, perm, or straighten your hair, you trigger a chemical change. These processes rearrange the chemical bonds, changing the very nature of your hair strands. It’s more than just a style update; it’s a molecular makeover.
Sweet Lessons: Sugar Under the Microscope
White sugar is crystalline, sweet, and dissolves quickly in warm water. These are physical properties. No magic needed—just observation.
Melting sugar is a neat physical change. It becomes liquid but remains sugar.
Heat the sugar longer, though, and it darkens, smells different, and eventually burns. These are signs of a chemical change. Now you’re witnessing sugar’s chemical properties—flammability and combustibility—in action.
Why Bother Differentiating?
If you can tell these apart, you get a clearer understanding of everyday life and the science behind it.
Scientists use this distinction to study substances and predict how they behave. Chefs unknowingly perform chemical changes all the time when baking or grilling. Hairdressers manipulate chemical bonds for stylish effects. Even recycling depends on knowing what’s a physical change versus what’s a chemical one.
How Can You Tell Them Apart Quickly?
| Aspect | Physical Property | Chemical Property | Physical Change | Chemical Change |
|---|---|---|---|---|
| Observation | Can be observed without altering substance | Requires changing substance to observe | Changes shape or state, substance remains | Substance becomes new substance |
| Examples | Color, texture, melting point | Combustibility, reactivity | Cutting, melting, freezing | Burning, rusting, baking |
| Signs | No change in substance | Change occurs | No change in chemical makeup | Color, odor, gas, temp changes |
Final Thoughts: Remember These Quick Tips
- Physical property/change = NO new substance.
- Chemical property/change = NEW substance forms or can form.
- Look for clues: color, bubbles, smell, or heat can tell you if chemical changes are happening.
- Try simple examples like sugar heating or haircuts to spot differences.
Next time you see a change, pause and ask: Is this just a new shape or a whole new thing? Understanding this simple question unlocks much of chemistry’s magic in our everyday world.
What is the key difference between a physical property and a chemical property?
A physical property can be observed without changing the substance. A chemical property requires altering the substance to see the property.
How can you tell if a change is physical or chemical?
If the substance’s identity stays the same but its form changes, it is a physical change. If a new substance forms, it is a chemical change.
Can you give a simple example of a physical property versus a chemical property?
- Physical property: hair color.
- Chemical property: how easily hair burns.
What are signs that a chemical change has happened?
Look for color or odor changes, gas bubbles, temperature shifts unrelated to heating sources, or sounds and light being produced.
Why is cutting hair a physical change but dyeing it a chemical change?
Cutting hair only changes its shape. Dyeing hair rearranges its chemical bonds, creating a new substance.


Leave a Comment