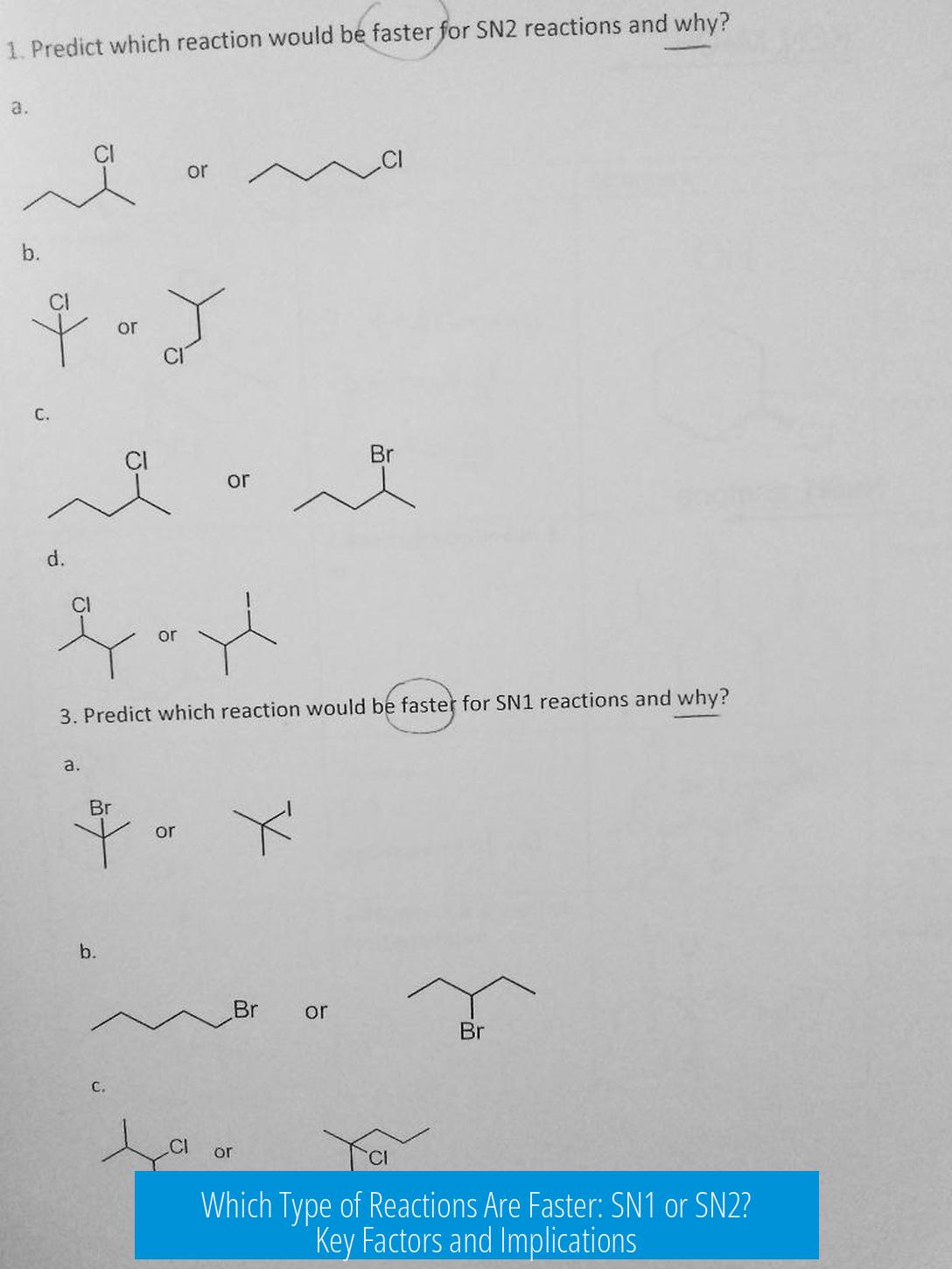
Which Type of Reaction is Faster: SN1 or SN2? Why?

The speed comparison between SN1 and SN2 reactions depends on the reaction conditions and substrate; neither is universally faster. Several factors interplay to determine which mechanism proceeds more rapidly.
1. No Universal Speed Order
There is no absolute rule that SN1 or SN2 reactions are always faster. The reaction rates depend on the substrate structure, solvent, nucleophile, and temperature. Under some conditions, SN1 dominates; under others, SN2 is quicker. Both mechanisms may compete simultaneously.
2. Key Factors Influencing Reaction Rates

- Substrate: Tertiary alkyl halides favor SN1; primary substrates favor SN2.
- Nucleophile: Strong nucleophiles accelerate SN2 reactions but have less effect on SN1.
- Solvent: Polar protic solvents stabilize carbocations, facilitating SN1. Polar aprotic solvents favor SN2 by increasing nucleophile strength.
- Temperature: Higher temperature can affect both rates differently, depending on activation energies.
3. Mechanistic Complexity
SN2 follows a one-step, bimolecular concerted mechanism. This often makes it faster because bond-breaking and bond-making occur simultaneously. SN1 proceeds via a two-step process: first, the formation of a carbocation intermediate (slow rate-determining step), then nucleophilic attack. The multistep nature can reduce overall rate.
4. Reaction Order Clarification
The numeric ‘1’ or ‘2’ refers to kinetic order, not speed. SN1 is first order (depends solely on substrate concentration), and SN2 is second order (depends on both substrate and nucleophile concentration). This does not imply SN1 is faster; rather, it informs how rate changes with concentration.
5. Practical Implications
In practice, determining which reaction is faster requires analyzing substrate type and conditions. For example, SN2 is favored with primary halides and strong nucleophiles, often proceeding rapidly. SN1 is favored by tertiary substrates and polar solvents but may be slower due to carbocation formation.
| Factor | Effect on SN1 Rate | Effect on SN2 Rate |
|---|---|---|
| Substrate | Tertiary > Secondary > Primary | Primary > Secondary > Tertiary |
| Nucleophile | Little effect | Strong nucleophiles increase rate |
| Solvent | Polar protic favors | Polar aprotic favors |
| Temperature | Varies, often favors carbocation formation | Can increase reaction speed by increased collision frequency |
Key Takeaways
- Neither SN1 nor SN2 is universally faster; speed depends on substrate and reaction conditions.
- SN2 is often faster due to its one-step mechanism.
- SN1 involves carbocation formation, adding a rate-determining step and sometimes slowing the reaction.
- Reaction order (first or second) does not equate to reaction speed.
- Solvent, nucleophile strength, and temperature all critically impact rates.
Which type of reaction is generally faster, SN1 or SN2?
The speed cannot be universally stated. SN2 is often faster because it is a one-step process, but SN1 speed depends on other factors and can be faster in some cases.
Why is SN2 considered potentially faster than SN1?
SN2 involves a single step where the nucleophile attacks and the leaving group leaves simultaneously. SN1 involves two steps with an intermediate, which can slow the overall rate.
How do substrate and nucleophile affect the speed of SN1 and SN2 reactions?
Substrate structure and nucleophile strength impact rates. SN2 favors less hindered substrates and strong nucleophiles. SN1 favors stable carbocations, often from tertiary substrates.
Does the reaction order number in SN1 or SN2 indicate which is faster?
No. The numbers denote reaction order (SN1 is first order; SN2 is second order) and do not directly imply speed differences.
Can both SN1 and SN2 occur in the same reaction? Which will be faster then?
Yes, both mechanisms can compete. The faster one depends on reaction conditions, substrate, and reagents, making each case unique and requiring analysis.


Leave a Comment