Conversion of Alkene to Alcohol
Alkenes convert to alcohols through reactions that involve electrophilic activation. Because alkenes are weak nucleophiles, they need a strong electrophile or catalyst to facilitate the addition of a hydroxyl group.
Alkene Reactivity and Electrophilic Activation
Alkenes contain a carbon-carbon double bond with high electron density but do not readily react with weak electrophiles. Therefore, successful conversion to alcohol requires creating or using a sufficiently electrophilic species. This activation allows the alkene to form intermediates that ultimately yield the alcohol product.
Common Methods for Alkene to Alcohol Conversion

- Oxymercuration-Demercuration: Mercury(II) acetate serves as a strong electrophile. It adds to the alkene to form a mercurinium ion intermediate. Subsequent reduction with sodium borohydride replaces mercury with hydrogen, producing a Markovnikov alcohol without carbocation rearrangement.
- Hydroboration-Oxidation: Boron, with its empty p-orbital, acts electrophilically by adding across the double bond in an anti-Markovnikov manner. Following addition, oxidation with hydrogen peroxide converts the organoborane intermediate into an alcohol. Sodium borohydride is not used here since oxidation requires an oxidizing agent, typically H2O2.
- Acid-Catalyzed Hydration: Water alone cannot add to alkenes effectively. A strong acid like sulfuric acid protonates the alkene, forming a carbocation intermediate. Water then nucleophilically attacks this carbocation, generating the alcohol. However, this reaction is prone to carbocation rearrangements and often yields mixtures.
Reaction Conditions and Challenges
Direct addition of water to alkenes is thermodynamically and kinetically unfavorable due to entropy and alkene nucleophilicity. Extremely harsh conditions, such as pressures around 10,000 psi and temperatures near 600 °C, might allow the reaction but are rarely practical.
Using catalysts lowers energy barriers and improves selectivity. The choice of method depends on the desired regiochemistry, tolerance for rearrangements, and reaction conditions.
Summary of Methods

| Method | Electrophilic Activator | Markovnikov/Anti-Markovnikov | Advantages | Limitations |
|---|---|---|---|---|
| Oxymercuration-Demercuration | Mercury(II) acetate | Markovnikov | No rearrangement, mild conditions | Use of toxic mercury |
| Hydroboration-Oxidation | Boron (empty p-orbital) | Anti-Markovnikov | Regioselective, mild conditions | Requires careful control of oxidation step |
| Acid-Catalyzed Hydration | Proton from strong acid | Markovnikov | Simple reagents | Rearrangements common, less control |
- Alkenes need a sufficient electrophilic species to react.
- Oxymercuration and hydroboration provide regioselective access to alcohols.
- Acid hydration is simple but less controlled due to carbocation rearrangements.
- Direct water addition is unfavorable without catalysts or harsh conditions.
Turning Alkenes into Alcohols: The Chemistry Behind the Magic
If you’re wondering how to convert an alkene to an alcohol, the answer lies in activating the alkene with a sufficiently powerful electrophile. That’s chemistry’s way of saying, “Alkenes aren’t very reactive on their own, so you need a strong partner to pull it off.”
Let’s break this down — because understanding this makes organic chemistry less about magic and more about smart moves. Alkenes are double-bonded carbons, but their nucleophilicity (their craving to donate electrons) is pretty weak. Hence, they don’t just react with anything. They require an electrophile — a species that loves electrons and is willing to grab the electron-rich double bond.
Why Can’t You Just Add Water?
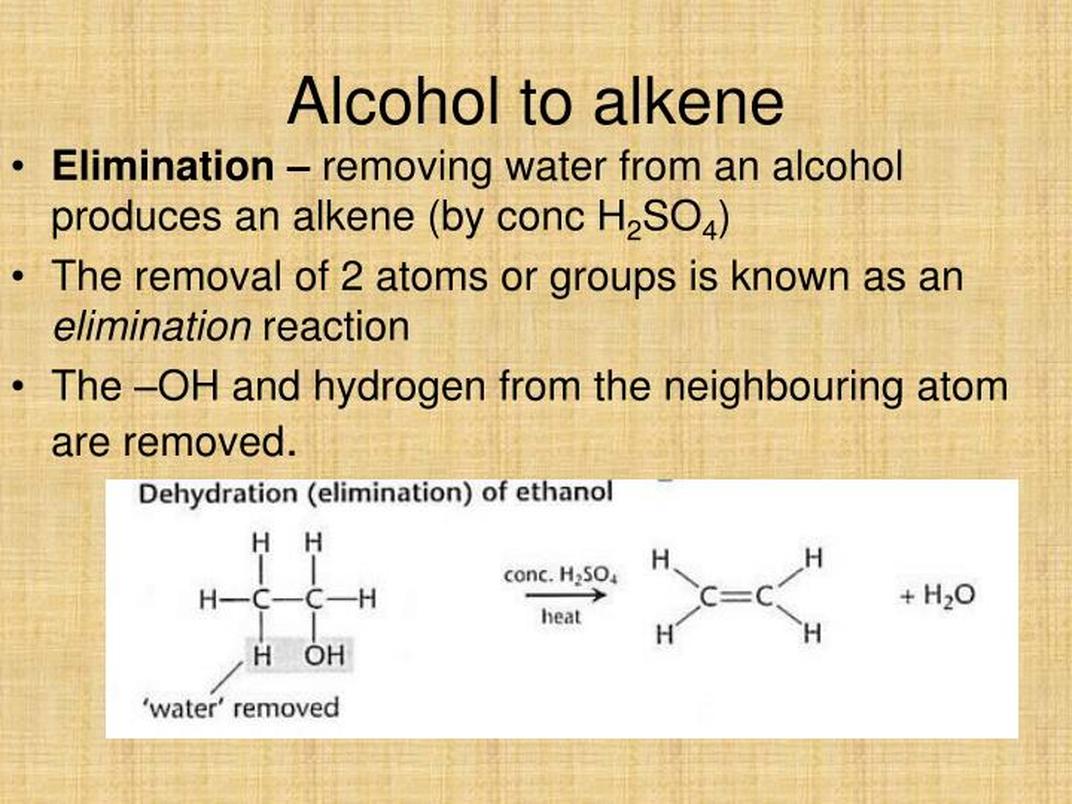
You might guess, “Why not simply add water to an alkene and get an alcohol?” Good thought, but water is a neutral molecule and not electrophilic enough to attack the alkene. On paper, this seems straightforward, but in reality, things are trickier because of entropy and the alkene’s low nucleophilicity. This means the reaction is very slow or unfavorable unless you crank up the heat and pressure — like seriously intense conditions (think 10,000 psi and 600 °C) which are, frankly, impractical for most labs.
If you’re patient and have a knack for braving nuclear reactor conditions, good for you. Otherwise, catalysis is the smarter, easier route.
The Classic Methods: Oxymercuration-Demercuration and Hydroboration-Oxidation
Luckily, chemists came up with clever methods that make this process more manageable. Here’s the lowdown on two popular, widely used reactions:
- Oxymercuration-Demercuration: Think of mercury acetate as a superstar electrophile that’s just powerful enough to activate the alkene.
- Hydroboration-Oxidation: Here, the empty p-orbital on boron acts as the shrewd electrophile. It swoops in, binding to the alkene, setting the stage for oxidation by hydrogen peroxide to finalize the transformation.
Both methods convert alkenes to alcohols without the crazy rearrangements or side reactions that make your life miserable.
Deep Dive: Oxymercuration-Demercuration
In oxymercuration, mercury acetate is the star. The Hg(OAc)2 attacks the alkene’s double bond, creating a mercurinium ion intermediate. This positively charged species is highly electrophilic and allows water to sneak in and add across the double bond.
Once water adds, a reduction step called demercuration replaces the mercury with hydrogen, leaving you with a stable alcohol.
This reaction is a favorite because it’s mild and effective, yielding predominantly Markovnikov addition products without carbocation rearrangements. In other words, the alcohol ends up on the more substituted carbon, which often aligns with synthetic goals.
Hydroboration-Oxidation: The Softer Approach
Hydroboration-oxidation is a two-step process. First, borane (BH3) or a boron-containing reagent binds to the alkene, with boron attaching to the less substituted carbon due to steric reasons. That’s where the empty p-orbital on boron shines as the electrophile.
Next comes oxidation, but here’s a crucial detail: you must use hydrogen peroxide or a similar oxidant, not sodium borohydride (NaBH4). Hydrogen peroxide swaps the boron for a hydroxyl group, giving an alcohol that follows anti-Markovnikov addition — meaning the OH is on the less substituted carbon.
This method is highly selective and avoids carbocation intermediates, reducing unwanted rearrangements. Bonus: It’s perfect when you want the alcohol on the less crowded side of your molecule.
Acid-Catalyzed Hydration: The Wild Card
If you don’t mind a bit of chaos, acid-catalyzed hydration can add water across the alkene.
This method starts with protonation of the alkene by a strong acid like sulfuric acid, forming a carbocation intermediate. Water then attacks this carbocation to produce the alcohol.
Sounds simple, right? Well, kind of. The problem is this reaction is uncontrolled and often leads to rearrangements — meaning your product might be a mixture of unexpected structures, making purification and interpreting your results a headache. It’s chemist-speak for “messy, but sometimes necessary.”
Direct Hydration? Only With Serious Muscle
Adding water directly without catalysts or harsh conditions generally won’t work. If the alkene is highly strained or has electron-withdrawing groups (like ene-ones), the reaction can proceed more easily, but that’s the exception, not the rule.
To force direct hydration, you need high pressures and temperatures (e.g., 10,000 psi and 600 °C). Those aren’t answers you want unless you’re the action-hero chemist in an industrial lab movie.
Why Electrophiles Matter So Much?
It all boils down to reactivity. Alkenes’ double bonds buzz with electrons but aren’t eager to attack. To transform that double bond into an alcohol, you need an electrophilic partner to activate it.
Mercury acetate and boron compounds bring just the right dose of electrophilicity without causing too much mayhem. On the other hand, water alone or weak electrophiles let the reaction sputter or explode into unwanted products.
In Summary: Which Method Should You Choose?
| Method | Electrophile & Activation | Pros & Cons |
|---|---|---|
| Oxymercuration-Demercuration | Mercury acetate | Effective, mild; gives Markovnikov alcohols; minimal rearrangement |
| Hydroboration-Oxidation | Boron compound + oxidant (H2O2) | Anti-Markovnikov; high selectivity; no rearrangements; peroxide required |
| Acid-Catalyzed Hydration | Protonation by strong acid (e.g. H2SO4) | Uncontrolled; rearrangements common; simple reagents |
| Direct Hydration | None (water only) | Very harsh conditions; rare; impractical in most labs |
Pretty clear, right? You pick your route based on the exact alcohol you want—and how much chaos you’re willing to tolerate.
Got a Strained Alkene or Electron-Withdrawing Groups? Bonus Points!
If your alkene is “strained” or has groups like ketones attached (eno-ones), then direct hydration or simpler methods might actually work better. The strain or electron-deficiency activates the alkene more naturally. Chemistry does throw you these bonus challenges.
Final Word: From Alkene to Alcohol, It’s All About Activation
Want to turn your alkene into an alcohol? Remember this: alkenes need to be *activated* by a strong electrophile. That could be mercury acetate, boron compounds, or even a strong acid. Or you brute-force it with pressure and heat, but that’s only if you like drama.
Chemistry isn’t just memorizing reactions; it’s about understanding why certain paths shine while others fizzle. So next time you see an alkene, think, “How am I going to seduce that double bond into becoming an alcohol?” and pick your partner accordingly.
How does oxymercuration-demercuration convert alkenes to alcohols?
Mercury acetate acts as a strong electrophile, activating the alkene. It adds across the double bond, forming an organomercury intermediate. Demercuration then replaces mercury with a hydroxyl group, yielding the alcohol.
Why is hydroboration-oxidation preferred over direct use of sodium borohydride?
Hydroboration uses boron’s empty p-orbital as an electrophile to activate the alkene. Then, oxidation with hydrogen peroxide converts the organoborane to the alcohol. Sodium borohydride does not oxidize the intermediate.
What limits the use of acid-catalyzed hydration for making alcohols from alkenes?
This method involves protonation of the alkene to form a carbocation, which then reacts with water. It often leads to rearranged products and is less controlled. The reaction can be unpredictable due to carbocation stability.
Can alkenes be directly hydrated with water without catalysts?
Direct water addition is unfavorable because water is not electrophilic enough and entropy works against the reaction. It may occur under extreme conditions like very high pressure and temperature but is rare and impractical without catalysts.
What role do catalysts and conditions play in alkene to alcohol conversions?
Catalysts activate alkenes by enhancing electrophilicity, enabling hydration reactions under milder conditions. Harsh conditions like 10,000 psi and 600°C might force the reaction, but catalysts offer a more practical approach to efficient conversion.





Leave a Comment