Understanding Benzenecarboxylic Acids
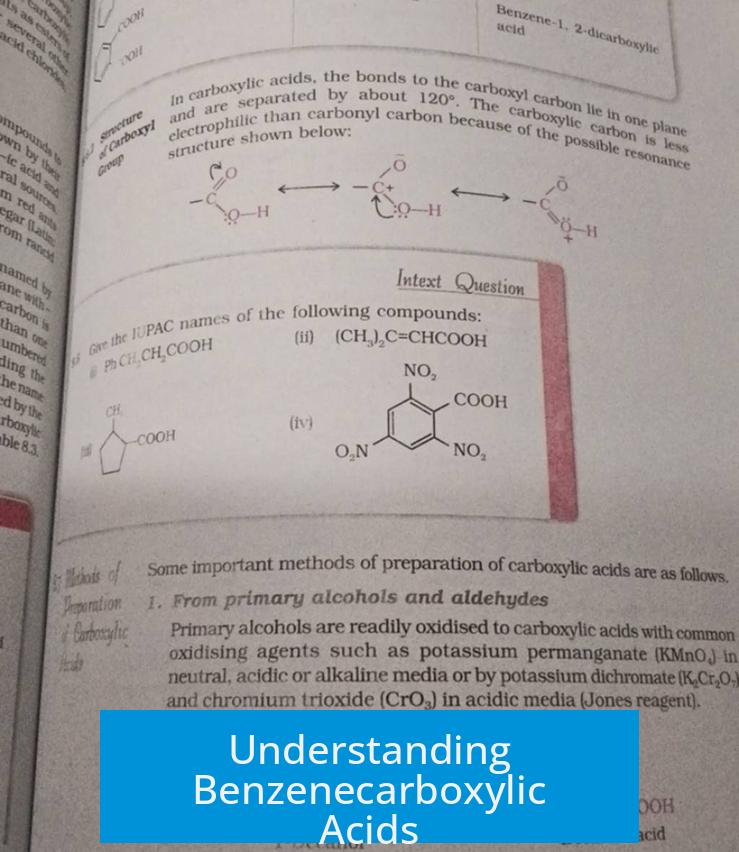
Benzenecarboxylic acids, such as benzoic acid, feature a conjugated system formed by the benzene ring and the carboxylic acid group. This conjugation creates a unified pi system that influences the compound’s properties and reactivity.
Conjugation in Benzenecarboxylic Acid
The defining characteristic of benzenecarboxylic acids is the conjugation between the aromatic ring and the carboxyl group. The π-electrons of the benzene ring interact directly with the π-system of the carboxylic acid. This extended conjugation stabilizes the molecule and affects its electronic distribution.
Electronic Effects: Mesomeric versus Inductive
Benzenecarboxylic acids exhibit both mesomeric (resonance) and inductive effects from substituents. Mesomeric effects (+M or -M) involve delocalization of electrons through resonance, which is typically stronger than inductive effects (-I or +I), which operate by sigma bonds through electron withdrawal or donation.
- Groups featuring +M and -I effects often act as overall activating substituents on the aromatic ring.
- The carboxyl group (-COOH) primarily exerts a -I effect, withdrawing electron density via sigma bonds, decreasing electron density on the ring.
- However, the resonance interaction of the carboxyl group with the benzene ring enhances delocalization, modifying the ring’s electronic nature.
Impact on Electrophilic Substitution
The combined electronic effects influence electrophilic aromatic substitution reactions. The electron-withdrawing carboxyl group deactivates the benzene ring toward electrophiles, especially at the ortho and para positions. This effect stems from the -I inductive withdrawal and resonance stabilization of the substituent, which reduces ring electron density.
This behavior explains the lower reactivity of benzenecarboxylic acids in such reactions compared to unsubstituted benzene. The meta position tends to be favored for substitution since it is less destabilized by resonance with the carboxyl group.
Delocalization Predominance in Benzenecarboxylic Acids
In benzenecarboxylic acids, the delocalization is unified between the benzene ring and the carboxyl group. Rather than separate competing effects, the π electrons are part of an extended conjugated system. This extended conjugation impacts stability, acidity, and reactivity patterns.
Key Takeaways
- Benzenecarboxylic acids feature a single, conjugated pi system combining the benzene ring and carboxyl group.
- Mesomeric (resonance) effects typically dominate over inductive effects in determining electronic behavior.
- The carboxyl group withdraws electron density inductively, deactivating the aromatic ring in electrophilic substitution.
- Electrophilic substitution occurs preferentially at the meta position due to these electronic influences.
- Delocalization stabilizes the molecule and affects its chemical properties and reactivity profiles.
What does conjugation mean in benzenecarboxylic acids?
In benzenecarboxylic acids, the benzene ring and the carboxylic acid group share a conjugated pi system. This means their electrons are delocalized over both parts, creating one continuous pi system.
How do mesomeric and inductive effects influence benzenecarboxylic acids?
Mesomeric effects are stronger than inductive effects. Groups with both +M (mesomeric) and -I (inductive) effects act mainly as activating groups in reactions.
Why is the mesomeric effect more significant than the inductive effect here?
The mesomeric effect involves electron delocalization through resonance, stabilizing the system. Inductive effects involve electron withdrawal or donation through sigma bonds, which is weaker.
How do benzenecarboxylic acids affect electrophilic substitution on the benzene ring?
The carboxyl group usually deactivates the ring and directs substitution to the meta position. This is due to its electron-withdrawing nature via resonance and induction.
What type of delocalization occurs in benzenecarboxylic acids?
The benzene ring and carboxylic acid group form a combined pi system. Delocalization extends over both, influencing the acid’s reactivity and properties.




Leave a Comment