Understanding Constitutional Isomers
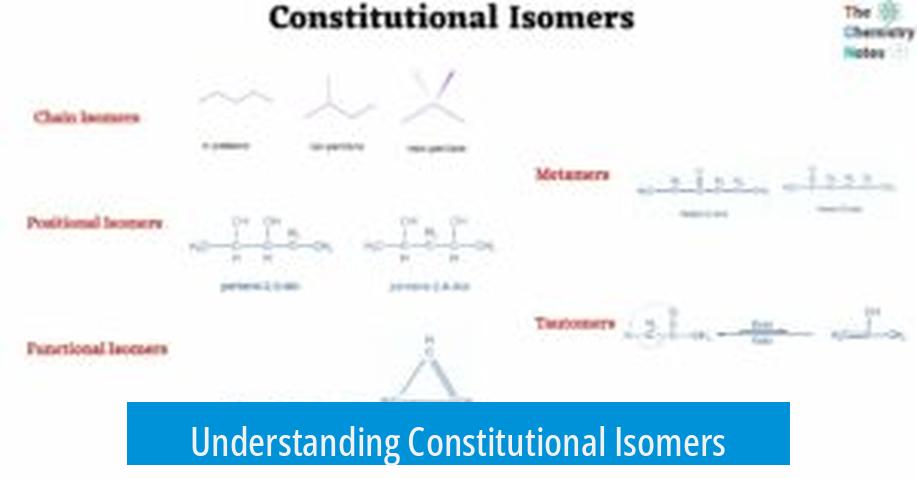
Constitutional isomers are compounds sharing the same molecular formula but differing in the connectivity of their atoms. They provide multiple structural possibilities for a given molecular formula. This distinction in connectivity leads to varied chemical and physical properties despite identical formulas.
No Simple Formula for Enumeration
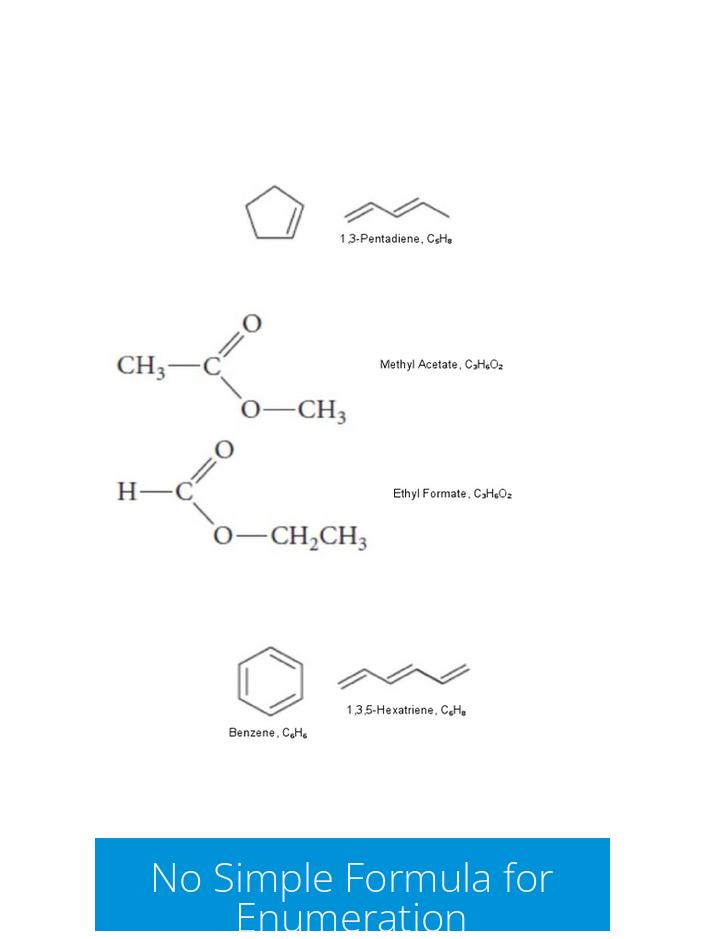
There is no straightforward or convenient formula to predict the total number of constitutional isomers for a molecular formula. The diversity of possible structures grows rapidly with molecular size. Thus, enumeration requires a systematic method or computational tools rather than applying a direct formula.
Systematic Enumeration Approach

To find constitutional isomers, one can proceed stepwise:
- Begin with the straight-chain structure corresponding to the formula.
- Reduce the number of carbon atoms in the main chain by one.
- Identify all isomers formed by adding a single branch to this shorter chain.
- Repeat this process, progressively shortening the main chain and adding branches until all possibilities are examined.
This method ensures no structures are overlooked while allowing classification by branching patterns.
Role of Degrees of Unsaturation
Degrees of unsaturation (DoU) indicate the number of rings and/or multiply bonded atoms in a molecule. It is calculated from the molecular formula and guides structural considerations:
- Calculate DoU to understand if rings or double bonds can be present.
- If DoU equals zero, exclude consideration of rings and double bonds, focusing only on saturated structures.
- This reduces the complexity of isomer enumeration, narrowing the search.
Practical Applications
Understanding constitutional isomers aids in:
- Structural determination from empirical formulas.
- Designing synthesis routes targeting specific isomers.
- Predicting chemical properties based on connectivity differences.
Key Takeaways
- Constitutional isomers have identical molecular formulas but differ in atom connectivity.
- No direct formula exists to count all possible isomers.
- Systematic, stepwise enumeration helps find all isomers.
- Degrees of unsaturation direct structural possibilities and simplify enumeration.
- Understanding these isomers is crucial for chemical analysis and synthesis planning.
Unlocking the Mysteries of Constitutional Isomers: A Step-by-Step Guide
What exactly are constitutional isomers? In the simplest terms, they’re molecules sharing the same molecular formula but differing in how their atoms connect. Unlike twins separated at birth, these are chemical siblings with distinct blueprints. They look similar on paper but behave differently in the lab and beyond.
Now here’s the kicker: there’s no shortcut formula to list out all constitutional isomers. That’s right—no magic equation or instant calculator can tell you how many exist for a given formula. It’s a puzzle that demands patience, strategy, and a bit of chemistry know-how.
Imagine you’re sorting through a box of puzzle pieces with the same number of pieces but multiple ways to fit them together. This is the core of the challenge when counting or drawing constitutional isomers.
The Challenge: No Convenient Formula Exists
Many students and even seasoned chemists get thrown off because, unlike combinations or permutations in math, constitutional isomers don’t follow an easy pattern. According to reliable sources, there is no convenient formula that perfectly predicts the number of constitutional isomers for every molecular formula.
Why is that? Because the number depends heavily on the connectivity possibilities, which grow exponentially with more atoms. Some molecules can have just one or two isomers, while others explode into dozens or even hundreds of variants. So, chatting with your calculator or typing a quick query won’t cut it.
How to Tame the Beast: Methodical Enumeration
So if no formula exists, what’s the game plan? The answer is methodical enumeration: a stepwise, often manual (or computational) approach to generate every possible structure.
Picture this as carefully putting together building blocks, starting from a simple model and adding complexity step by step. For example, start with the straight-chain isomer—the one where all carbon atoms form a single line.
Then, “drop one carbon” and consider all possible configurations with a single branch. Repeat by gradually reducing the chain length and increasing branching. This process ensures you don’t miss any structural variants and systematically cover the entire landscape.
Computers can speed this up. But the key principle is clear: you either count by hand or get a computer to count for you—but always with structure-driven logic.
Degrees of Unsaturation: Your Secret Weapon for Structural Clues
This might sound fancy, but recognizing degrees of unsaturation can be a lifesaver here. Degrees of unsaturation tell you how many rings or double bonds an isomer can have, based on the molecular formula.
Calculate this before you draw a single bond. For example, if the degrees of unsaturation are zero—guess what? Rings and double bonds are off the table. This instantly narrows down possibilities.
Think of degrees of unsaturation as a traffic cop directing you where you can and can’t go structurally. It slashes down the guesswork and enhances accuracy in your enumeration process.
Practical Example: Counting Isomers of C4H10
Take butane and its isomers (C4H10). Since degrees of unsaturation are zero, you know straightaway there are no double bonds or rings.
By starting with the straight chain and then considering branched chains (one branch on carbon 2 or 3), you find precisely two isomers: n-butane and isobutane. No computer needed—just logic and systematic thinking.
So Why Does This Matter?
Understanding constitutional isomers isn’t just academic. It opens doors to grasping how molecules behave differently despite similar formulas. This knowledge impacts drug design, materials science, and even flavor and fragrance creation.
In pharmaceuticals, one isomer might be therapeutic while another is harmful. Missing even a single isomer could have serious consequences. So mastering the enumeration methods, guided by degrees of unsaturation, is vital.
Tips to Master Constitutional Isomers Enumeration
- Always calculate degrees of unsaturation first. It cuts down complexity dramatically.
- Use stepwise enumeration. Start with simple structures and add complexity gradually.
- Draw out each isomer. Visualizing helps avoid duplicate structures and logical slips.
- Use molecular modeling software if stuck. Some programs auto-generate isomers to verify your work.
- Practice with small molecules. Build confidence on manageable examples before tackling big formulas.
Is This Process Ever Fun?
It depends. Some find this logical puzzle exhilarating, like solving a mystery with your chemical toolbox. Others may want to wring their hands. But here’s the positive spin: once you get the hang of it, this enumeration skill sharpens your molecular intuition.
Plus, it impresses chemists around the world, because you’re playing with molecules at their core—not just reciting formulas but discovering molecular identities.
In conclusion, constitutional isomers defy any easy formula for counting or listing them. They demand a methodical, patient approach, starting from fundamental principles. Calculating degrees of unsaturation gives you a crucial edge, helping rule in or out structural features. Then, work stepwise from simple chains to complicated branches. It’s a journey of chemical discovery, revealing how the same atoms can arrange to tell very different stories.
Next time you see a molecular formula, ask yourself: How many different structures could hide behind these letters and numbers? And then enjoy the detective work that is chemical isomerism.
What is the best way to find all constitutional isomers for a given molecular formula?
You need to list them methodically. Start with the straight-chain isomer. Then find all isomers with one branch, then two, and so on, reducing carbons to vary branching.
Is there a formula to directly calculate the number of constitutional isomers?
No. There isn’t a simple formula for this. The process requires systematic enumeration or computer algorithms to list all possible isomers.
How does degrees of unsaturation help in determining isomers?
Degrees of unsaturation limit your options. For example, if it is zero, you can exclude rings and double bonds. This narrows down possible structures.
Why is the stepwise branching approach useful in isomer enumeration?
It structures the search. By starting with a straight chain and then adding branches step by step, you avoid missing possible isomers during manual or computer analysis.




Leave a Comment