Crystals of Benzil: Synthesis and Characteristics

Benzil forms as light yellow colored crystalline solid through the oxidation of benzoin. The typical synthesis involves oxidizing benzoin using a strong oxidizing agent. In this specific procedure, concentrated 67% nitric acid acts as the oxidizer, converting the secondary alcohol group in benzoin into a diketone structure in benzil.
Formation of Benzil via Benzoin Oxidation
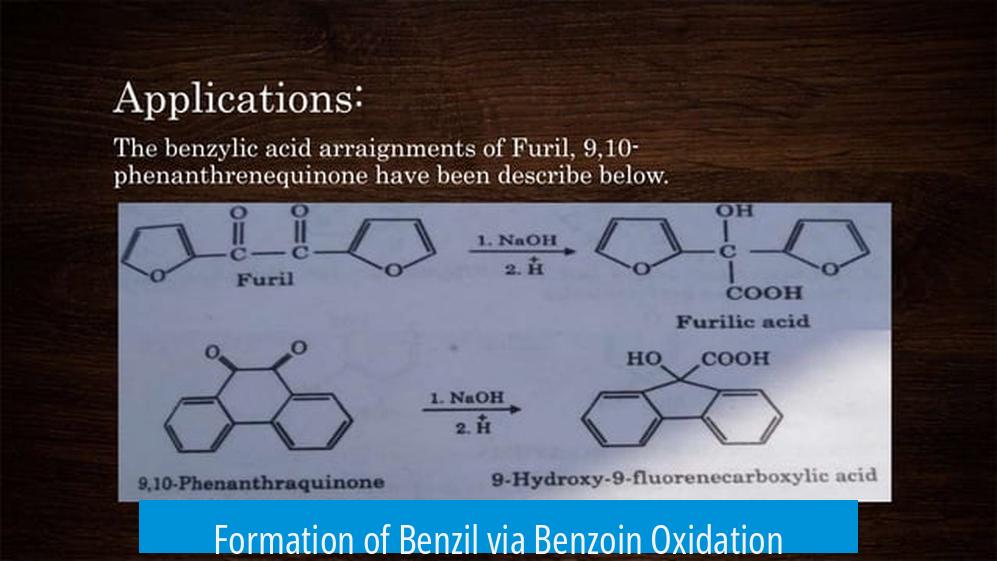
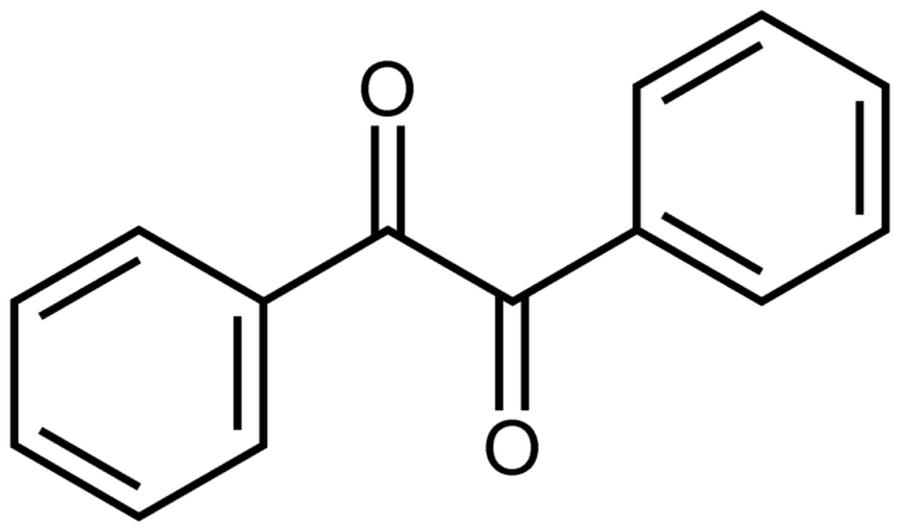
Benzil (1,2-diphenylethane-1,2-dione) results from the selective oxidation of benzoin. The reaction transforms the hydroxyl and adjacent hydrogen groups into two carbonyl groups (C=O), producing a diketone. The use of concentrated nitric acid is effective for this transformation because of its strong oxidizing power. It must be handled carefully due to its corrosive nature.
Physical Characteristics of Benzil Crystals
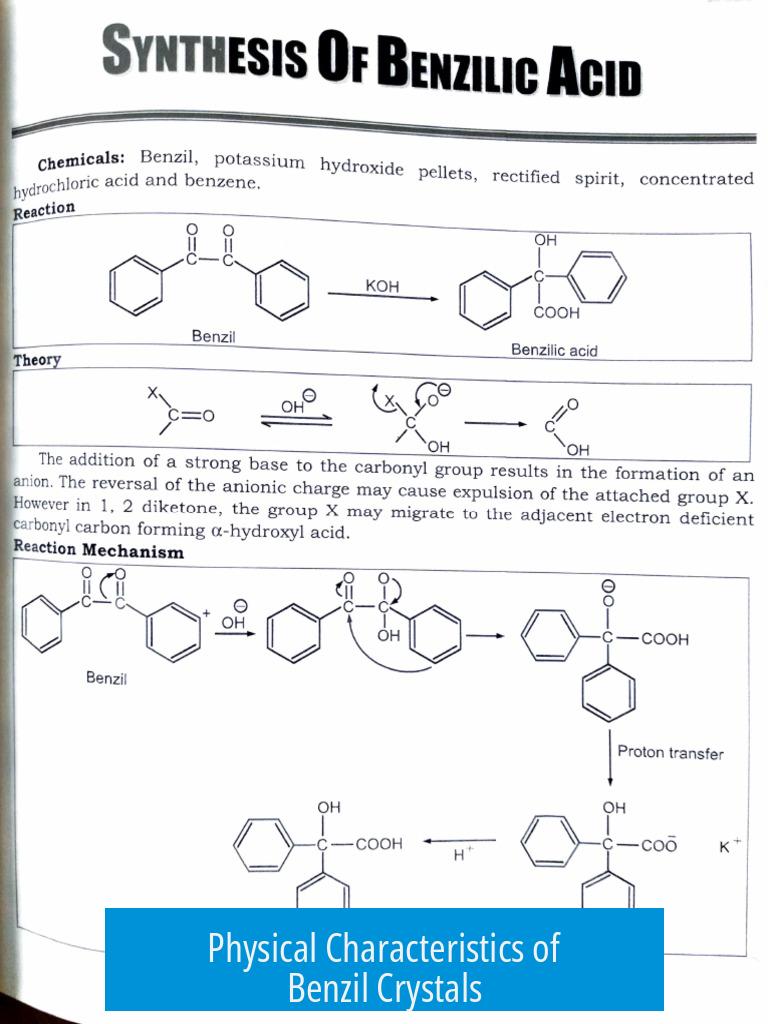
The resulting benzil crystallizes as a pale yellow solid. Crystals should ideally be well-formed and dry, showing sharp edges and a crystalline habit. The yellow color differentiates benzil from the white or colorless benzoin precursor.
During lab preparations, incomplete drying or inefficient filtration may leave the crystals wet with residual water. Such “wet” benzil may appear clumpy or resemble scrambled eggs, which can affect the crystal appearance and quality. Proper vacuum or suction filtration helps remove moisture, enhancing crystal purity and formation.

Practical Tips for Crystal Formation
- Use vacuum or suction filtration to dry crystals thoroughly.
- Control reaction temperature to improve crystal quality.
- Allow slow cooling to enhance crystal size and definition.
- Handle nitric acid with protective equipment to ensure safety.
Video Demonstration and Feedback Request
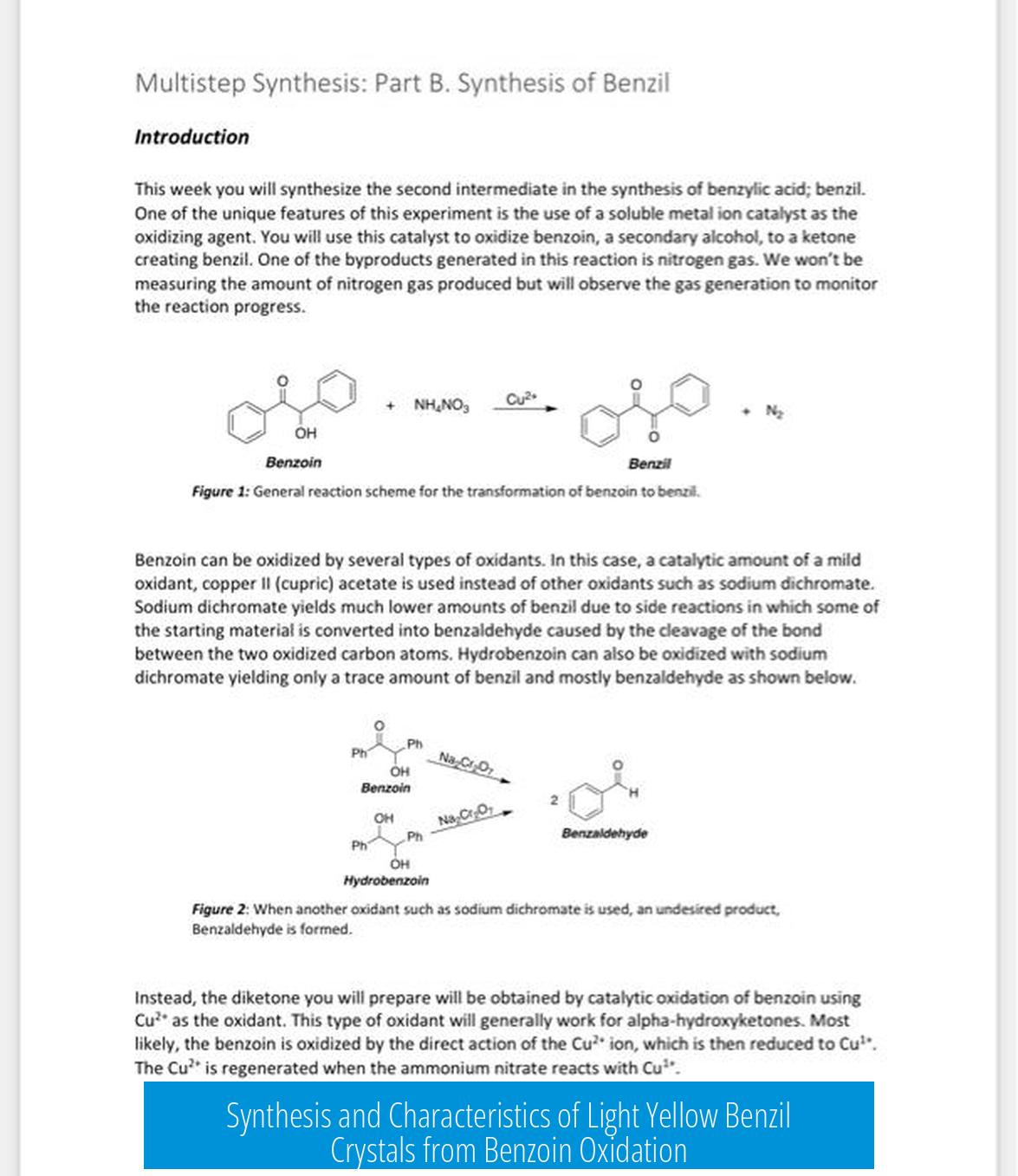
A video demonstrating this synthesis method is available here. Viewers are encouraged to observe the practical steps involved, including the handling of reagents, oxidation process, and crystallization technique. Constructive reviews can help improve practical understanding and technique.
Key Takeaways
- Benzil is synthesized by oxidizing benzoin with concentrated nitric acid.
- The product is a light yellow crystalline solid, distinct from benzoin.
- Proper drying and filtration are crucial for obtaining quality crystals.
- Crystals can appear wet or clumpy if filtration is inadequate.
- Video tutorials can aid in visualizing the synthesis steps and troubleshooting.
Crystals of Benzil: The Chemistry Behind the Light Yellow Gems
Ever wondered what those tiny, light yellow crystals in your chemistry lab really are? Meet benzil, a crystalline solid, often overlooked but oh so interesting. Benzil is a light yellow colored crystalline solid formed by the oxidation of benzoin, a transformation that’s as much about elegance as it is about chemistry precision.
If you’re picturing perfect, shiny crystals like in a jewelry box, think again. Benzil crystals can sometimes look more like… scrambled eggs. Yes, you read that right! So, why the discrepancy? It often boils down to the details in the synthesis and the drying process. But before we dive into the aesthetics, let’s break down what’s really going on.
How Are Benzil Crystals Made? The Oxidation Story
Benzil doesn’t just appear out of thin air. It’s formed when benzoin undergoes oxidation. In simple terms, oxidation here means the benzoin molecules lose electrons and transform into a new compound—benzil.
One effective way to achieve this oxidation is by using a concentrated 67% nitric acid solution as the oxidising agent. This method isn’t just textbook chemistry; it’s a tried and tested reaction that converts benzoin gently yet effectively into benzil. Concentrated nitric acid is a beast—handle it with care! But if used correctly, the reaction goes smoothly.
Why concentrate on this? Because choosing the right oxidizing agent impacts yield, purity, and ultimately, the crystals you get.
The “Scrambled Eggs” Phenomenon: What’s Going Wrong?
So, you’ve run the oxidation. You’re expecting perfect yellow crystals. But instead, reality bites: you get something more like a wet mess or even, as some students humorously complained, “forbidden scrambled eggs.” Is the chemistry broken?
Not really. The culprit is often the drying step. If your crystals remain wet, or the suction filtering wasn’t properly engaged during isolation, you get clumps and a soggy appearance instead of neat, shiny crystals.
Looks like it needs some suction filtering, probably still rather wet.
Vacuum filtration isn’t just a fancy name. It’s key to sucking away moisture and leaving you with dry, beautiful crystals ready for analysis or use. One student lamented, “My thing still had water in it cos my suction thingo wasn’t on properly,” which is a classic rookie mistake but an easy fix.
Pro tip: Ensure your suction filter is on and strong. If the end product looks like “cursed scrambled eggs,” it’s time to tweak your filtration settings and drying time.
The Beauty of Benzil Crystals: More Than Just Looks
Despite those scrambled egg comparisons, benzil crystals have a remarkable charm under the right conditions. They exhibit a soft, light yellow color—subtle yet distinct.
This light yellow hue isn’t just a random trait; it tells chemists they’re on target. Pure benzil has that distinctive shade, which indicates successful oxidation and minimal contamination.
While your setup might not produce perfectly glossy crystals every time, seeing those pale yellow solids is a sign you’re close to mastering the synthesis.
Learning from Experience: Tips and Practical Advice
- Video Learning: Watching someone else’s practical can save you hours of trial and error. I recorded my synthesis on video—I’ve linked it in the comments. Do watch and share your thoughts! Seeing each step helps clarify the tricky parts, especially handling nitric acid safely.
- Check Your Equipment: Suction filtering is not optional. It’s essential! Without proper filtration, wet crystals and “scrambled eggs” await.
- Patience Is Key: Allow adequate drying time. Rushing this leads to leftover moisture and poor crystal formation.
- Consult Trusted Sources: Someone suggested checking Vogel’s textbook for this preparation, and that’s solid advice. Vogel is a classic for good reason—clear, detailed protocols to boost your success.
Why Should You Care About Benzil Crystals?
You might be asking, “What’s so special about benzil anyway?” Beyond its interesting crystal form, benzil has uses as a reagent in organic synthesis and as a building block for pharmaceuticals and fragrances.
Understanding its synthesis is more than a lab chore—it’s a glimpse into organic transformations that power everything from medicines to materials. Plus, learning to turn benzoin into benzil means mastering oxidation, filtration, and crystal formation, which are fundamental chemistry skills.
Wrapping It Up: Are Benzil Crystals Worth the Effort?
If you enjoy crafting crystals and understanding how molecules jump from one state to another, benzil is a rewarding project. Sure, the results can sometimes look like “breakfast gone wrong,” but with proper technique, you’ll get those lovely, light yellow crystals that confirm you’ve cracked the reaction.
My suggestion? Watch the video, try the synthesis yourself, and tweak your suction filtering. It’s a satisfying journey from sticky benzoin to proud benzil crystals in your hands.
What say you? Are benzil crystals just chemical curiosities, or fascinating little trophies in the grand adventure of chemistry? Drop a comment, and let’s chat—especially if you have any tips on perfecting that suction filtering step!
And remember, no scrambled eggs allowed in your chemistry experiments!



Leave a Comment