Reaction of Grignard Reagent (MeMgBr) with Alcohol and Carbonyl
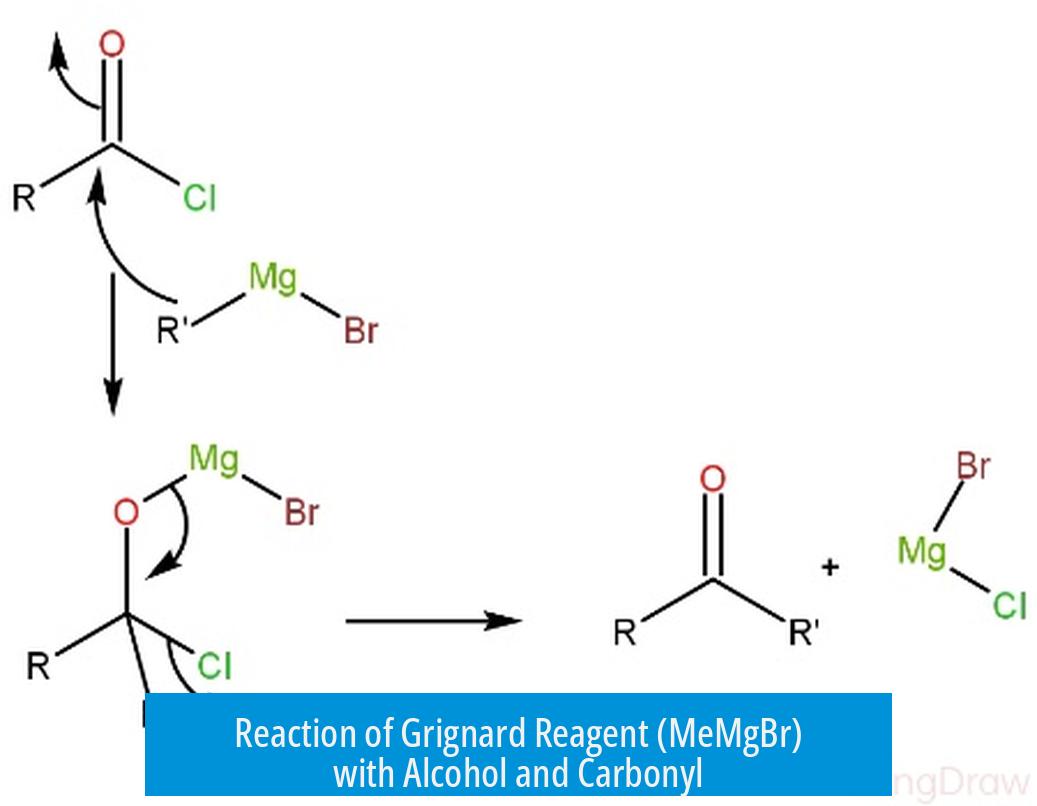
The Grignard reagent, MeMgBr, reacts with a compound containing both alcohol and carbonyl groups by first using one equivalent to deprotonate the alcohol, forming an alkoxide intermediate. The second equivalent then performs a nucleophilic attack on the carbonyl carbon, yielding the product where only one methyl group adds to the molecule.
Why Use Two Equivalents of MeMgBr?
- First Equivalent: The acidic proton of the alcohol is removed by MeMgBr. This converts the alcohol (–OH) into an alkoxide ion (–O^−), stabilizing the molecule and preventing unwanted side reactions.
- Second Equivalent: The second MeMgBr molecule attacks the electrophilic carbonyl carbon in the aldehyde or ketone. This nucleophilic addition forms a new carbon-carbon bond, leading to a secondary or tertiary alcohol after aqueous workup.
Using only one equivalent would result in incomplete reaction because the acidic proton of the alcohol consumes the initial MeMgBr, leaving none available for the carbonyl attack.
Nucleophilic Attack Site
The nucleophile targets the carbonyl carbon, not the oxygen of the alcohol. The carbonyl’s partial positive charge draws the methyl carbanion from MeMgBr, enabling bond formation. The alkoxide formed after deprotonation is less reactive and does not interfere with this process.
Stereochemical Considerations
The addition creates a new stereocenter. Predicting its configuration can often be guided by Felkin-Ahn’s model. This model considers steric and electronic factors to predict the preferred approach of the nucleophile and resulting stereochemistry.
Summary of Key Points
- One equivalent of MeMgBr deprotonates the alcohol, forming an alkoxide.
- The second equivalent adds nucleophilically to the carbonyl carbon.
- Only one carbon (from MeMgBr) is incorporated despite using two equivalents.
- The nucleophilic attack is on the carbonyl, not the alcohol.
- Felkin-Ahn’s model helps predict stereochemistry of the product.





Leave a Comment