How to Tell if a Substituent is Equatorial or Axial on Cyclohexane Rings
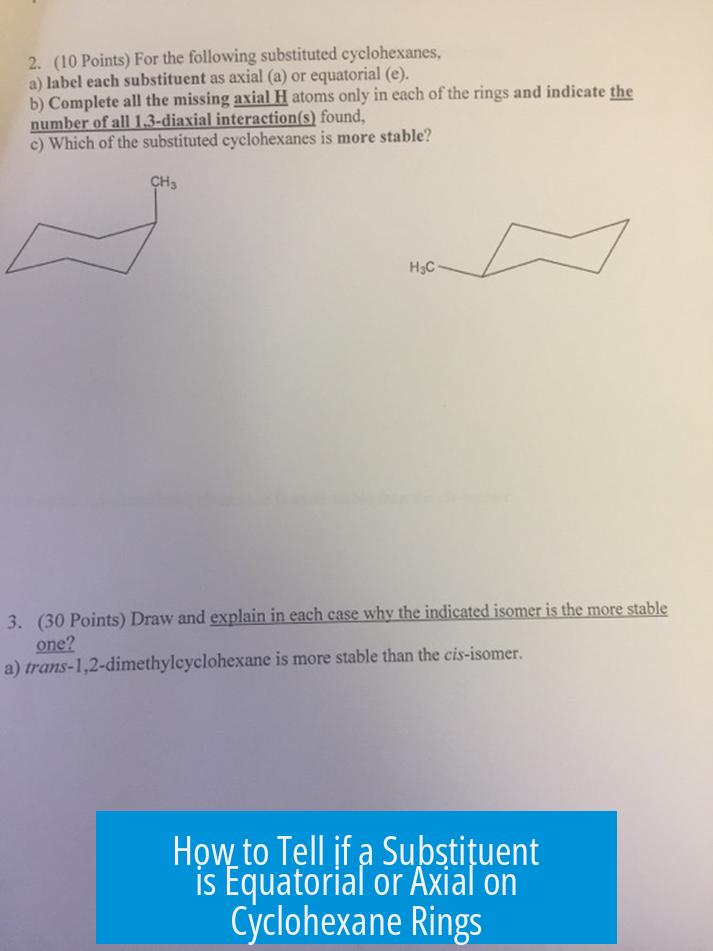
Equatorial substituents extend roughly parallel to the cyclohexane ring plane, while axial substituents point straight up or down relative to the ring’s center. This fundamental distinction helps identify their orientation once the substituent’s up or down direction is known.
Understanding Axial vs. Equatorial Positions
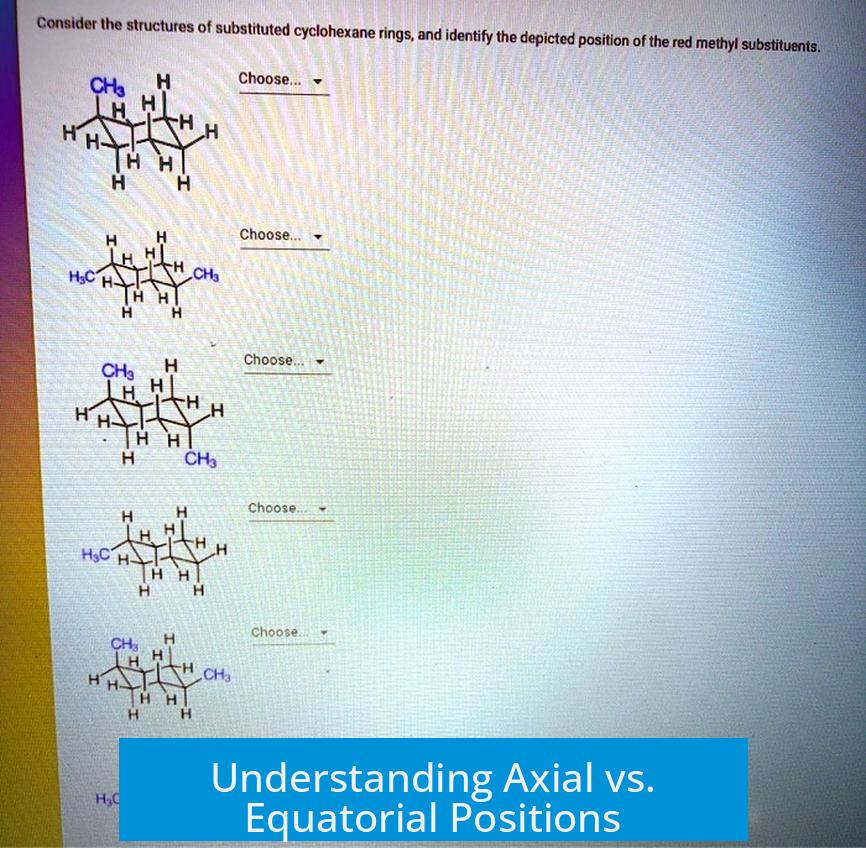
In a cyclohexane chair conformation, each carbon atom has two types of substituent positions:
- Axial: These substituents align perpendicular to the plane of the ring. They point either straight upward or straight downward, alternating direction around the ring.
- Equatorial: These substituents extend outward, roughly parallel to the ring bonds. They lie close to the plane but splay outward at an angle, following the ring’s contour.
To visualize this, draw the cyclohexane in chair form. Axial substituents are upright, while equatorial substituents run alongside the ring, usually parallel to visible carbon–carbon bonds.
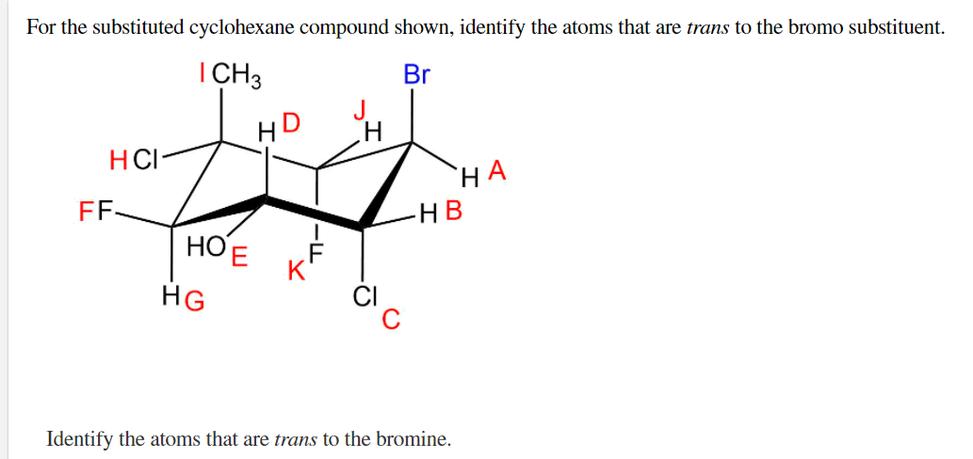
Practical Identification Tips
- Determine the ring conformation (usually chair form).
- Identify the carbon and the substituent direction (up or down) based on the stereochemistry.
- Check if the substituent is perpendicular (axial) or roughly parallel (equatorial) to the ring plane:
| Substituent Type | Orientation | Visual Cue |
|---|---|---|
| Axial | Straight up or straight down | Vertical to the ring plane |
| Equatorial | Parallel to ring bonds | Slanted, extending outward |
Why This Matters: Stability and Steric Effects
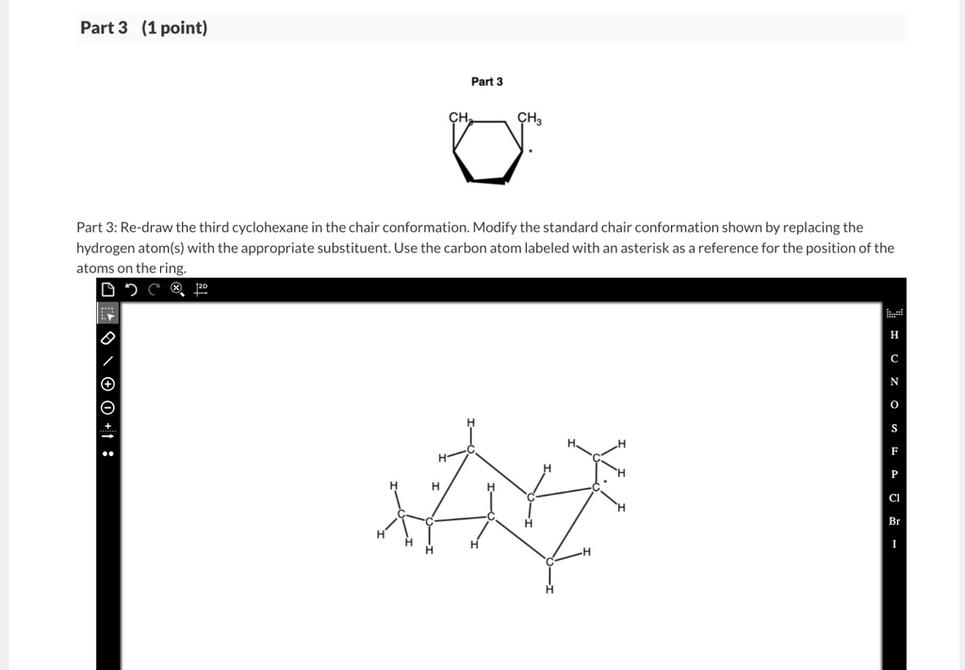
Large substituents are usually more stable in equatorial positions. Axial substituents face steric crowding with axial hydrogens on the same side of the ring, causing 1,3-diaxial interactions. Equatorial positions reduce this steric clash.
However, certain cases allow axial substituents to be stable if they participate in hydrogen bonding. Axial groups placed in 1,3-positions can interact via hydrogen bonding, which can offset steric strain and affect conformational preferences.
Summary
- Axial substituents are perpendicular to the ring, straight up or down.
- Equatorial substituents lie roughly parallel to ring bonds, extending outward.
- Bulky groups favor equatorial placement to avoid steric strain.
- Hydrogen bonding can sometimes stabilize axial substituents by reducing strain.
How To Tell If It Is Equatorial or Axial? The Cyclohexane Ring Mystery Solved!
If you’ve ever stared at a cyclohexane chair conformation and wondered, “Okay, I get how to spot up and down, but how do I figure out if a substituent is equatorial or axial?” — don’t worry, you’re not alone.
Axial substituents point straight up or straight down, perpendicular to the ring plane. Equatorial substituents, on the other hand, stretch out roughly parallel to the ring’s bonds, leaning slightly outward but not standing upright.
That’s the quick and dirty answer. But let’s unwrap this with a bit more flair and detail, so you never get caught scratching your head about positions on a cyclohexane ring again.
Axial Substituents: The Vertical Warriors
Imagine a cyclohexane chair with substituents as little flags. Axial flags wave straight up or straight down from the “center” of the ring.
Think of axial bonds as the ones that shoot almost perpendicular, like antennae, directly above or below the ring’s plane. They don’t mess around with the ring edges; they stand tall or dive deep.
This means, when you look at a chair conformation, anything sticking straight up or straight down from each carbon’s ring is axial. Simple visual cue, right?
Equatorial Substituents: The Outward Spreaders
Equatorial bonds are trickier. They don’t shoot straight; instead, they slide alongside the ring.
Here’s the catch: equatorial substituents run parallel to some bond within the cyclohexane ring. They point outward, slightly leaning up or down, but never vertically. You can think of these as branches growing horizontally from the ring, spreading their leaves sideways.
So, if your substituent is neither shooting straight up nor straight down but rather stretching flat or at a slight angle around the ring, you’ve got yourself an equatorial substituent.
Why Does This Even Matter? Stability, That’s Why.
Now you might ask, “Is it just a diagram thing, or does axial vs equatorial positioning really change anything?” Oh, it changes plenty.
Large substituents, like bulky groups or functional groups, want to hang out equatorially. Why? Because axial substituents often get cramped. This is due to 1,3-diaxial interactions — a fancy term meaning “things bump into each other in the axial positions.” Steric clashing, my friends.
Minimizing these bumps means more stable molecules. Don’t you want your molecule to be comfy and stable? Pretty sure yes.
So, the bulkier the R-group, the stronger the preference for it to be equatorial. Chemists use this rule of thumb to predict most stable chair conformers.
A Fun Exception: Hydrogen Bonding Sneaks In
Here’s a twist: not all axial substituents are unstable. Some can actually benefit from sitting axially because they get to form hydrogen bonds with neighbors.
When hydrogen bond donors and acceptors sit at beta positions (1,3,5 positions) and are forced in axial positions, they line up perfectly for hydrogen bonding. This bonding releases some energy and stabilizes the less-comfy axial position.
This means in some scenarios, axial substituents win in stability contests — against all odds.
So remember, bulky groups hate axial positions, but hydrogen bonding groups can get cozy even when axial. Chemistry loves drama!
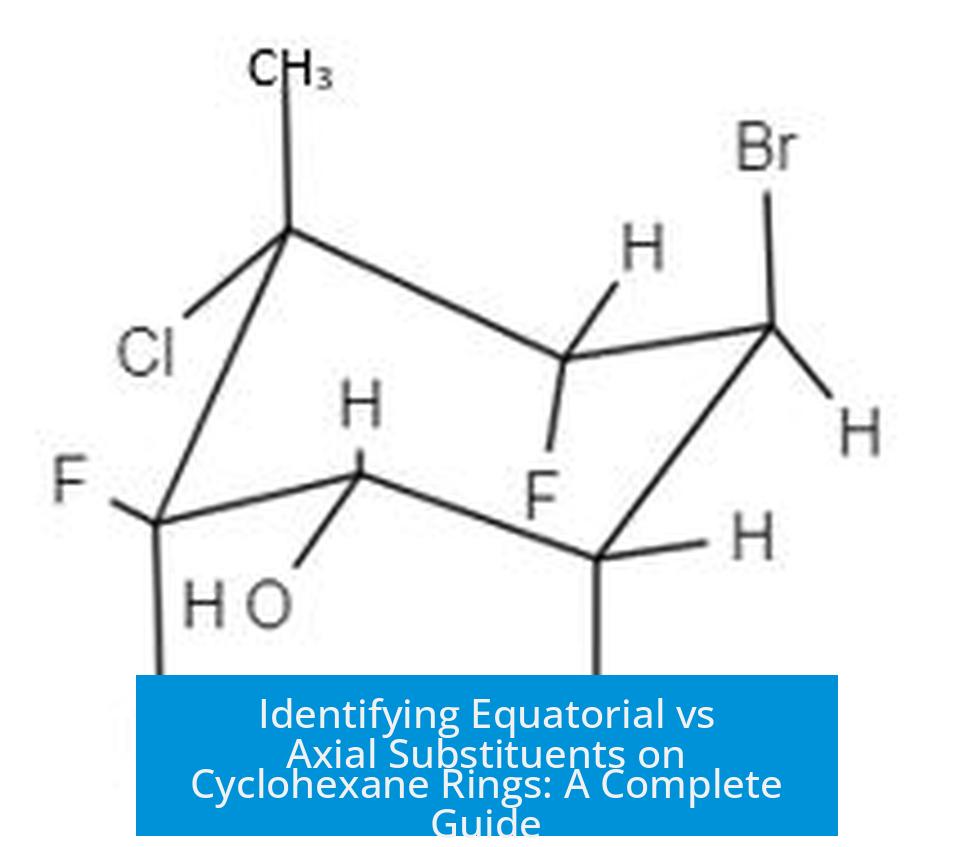
Real-Life Example: Hydroxyl Groups on Cyclohexane
Imagine two chairs:
- One with 2 axial hydroxyls
- Another with 4 axial hydroxyls
Logic says the chair with fewer axial bulky groups (2 axial OH) should be more stable, right? Usually yes.
But, if these hydroxyl groups can hydrogen bond effectively when axial, the chair with 4 axial OH might actually be more stable. It’s a balancing act of sterics vs bonding.
For more nitty-gritty, check out this great resource: Master Organic Chemistry on Substituted Cyclohexanes.
So, How Do You Know Equatorial vs Axial? A Little Checklist
- Look at the direction: Is the substituent pointing straight up or straight down? It’s axial.
- If it’s slanting outward: Usually parallel to a ring carbon bond, not vertical? That’s equatorial.
- Consider the size of your group: Bulky groups should prefer equatorial for more comfort.
- Think about bonding: If hydrogen bonding is possible and the groups align axially, stability might favor axial.
Why Care? Practical Tips for Chemistry Students and Scientists
Whether you’re a student tackling exam cyclohexanes or a professional designing organic molecules, identifying axial vs equatorial correctly is key.
It helps in:
- Predicting conformer stability
- Understanding reaction mechanisms
- Designing molecules with desired steric and electronic properties
If you ever get confused, try redrawing the molecule. Rotate the ring a bit. Visualize axial as vertical flags and equatorial as horizontal branches. Use 3D model kits if possible. Chemistry is a playground — make it fun.
Wrapping Up
So, to answer your question with style and clarity:
Axial substituents stand straight up or down — literally vertical to the cyclohexane ring plane, while equatorial substituents lie roughly parallel to the ring bonds, sticking out sideways. Bulky groups love equatorial seats due to less crowding, but hydrogen bonding can throw a curveball, making axial positions surprisingly cozy in some cases.
Next time you see a chair conformation, you won’t just think, “Up or down?” but, “Ah yes, equatorial or axial — and what story does this molecule tell?”
Now go earn that organic chemistry street cred!
How can I visually distinguish equatorial from axial substituents on a cyclohexane ring?
Axial substituents point straight up or straight down from the ring’s center. Equatorial substituents run roughly parallel to the ring bonds, extending outward at an angle. Look for vertical (axial) versus tilted (equatorial) positions.
Why are bulky groups usually found in the equatorial position?
Bulky groups in axial positions can cause steric clashes with nearby substituents. Equatorial positions offer more space, reducing crowding and making those conformers more stable.
Can axial substituents ever be more stable than equatorial ones?
Yes. When axial groups can form hydrogen bonds with other substituents in the right positions (like 1,3-diaxial), this stabilizes the axial conformation despite steric strain.
What does it mean that equatorial substituents align parallel to ring bonds?
This means the equatorial group follows the direction of one of the ring’s carbon-carbon bonds. It is not vertical but extends outward along the plane of the ring.
How do I decide the position of substituents in a cyclohexane chair drawing?
Mark axial substituents strictly vertical—either up or down from the ring. Place equatorial substituents roughly horizontal and angled slightly up or down, parallel to the adjacent ring bonds.




Leave a Comment