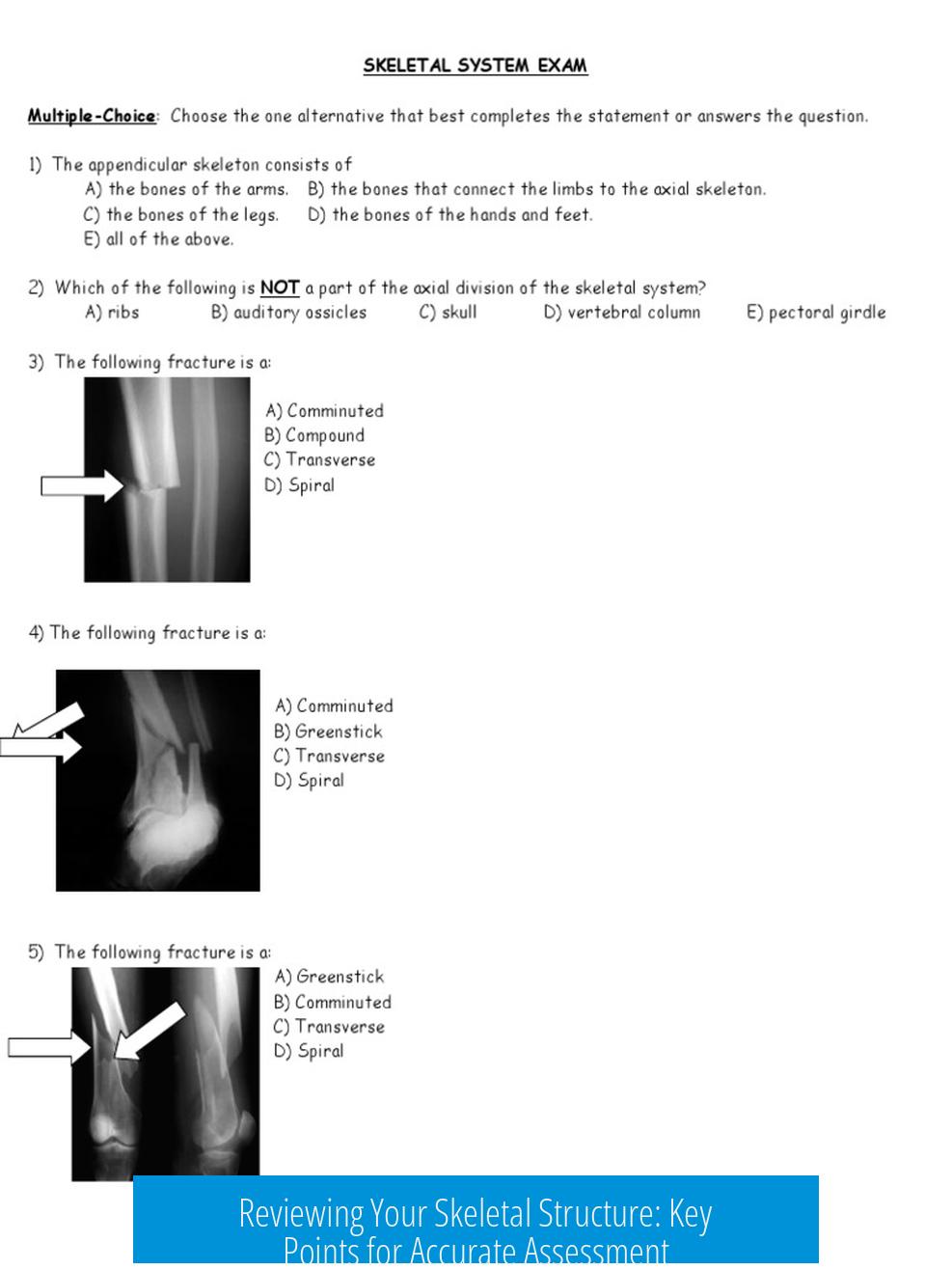
Reviewing Your Skeletal Structures: Key Points for Accuracy
If you just started drawing skeletal structures, it is crucial to double-check the longest carbon chain length, assign correct stereochemistry (E/Z), and follow IUPAC naming conventions precisely. These steps help ensure your work is accurate and understandable.
Counting the Longest Carbon Chain
Always recount the number of carbons in the longest continuous chain. For example, if your answer was five carbons but should be six, this changes the base name significantly.
- Verify chain length carefully before naming.
- Miscounting can lead to incorrect compound identification.
Identifying and Labeling Stereocenters
Double bonds can create stereocenters with E/Z configurations. Assign these where relevant:
- In compounds with double bonds, determine the priority of substituents based on Cahn–Ingold–Prelog rules.
- Label the double bonds as (E) or (Z) accordingly to indicate geometric isomers.
- Example: Compound #5 should be labeled (E)-3,5- because the double bond acts as a stereocenter.
Adhering to IUPAC Naming Conventions
Follow standardized rules for correct compound naming:
| Aspect | Correct Practice | Common Mistake |
|---|---|---|
| Naming chain length and position | Use pent-1-ene or hex-2-ene (prefix-number-ene) | Using “n-pentene” instead of pent-1-ene |
| Substituent numbering | Separate numbers with commas (3,5-diethyl) | Using dashes incorrectly (3-5-ethyl) |
| Hyphen and comma usage | Commas between numbers; hyphens between number and substituent | Mixing commas and hyphens incorrectly |
Specific Naming Tips
- For compound #3: Naming it simply as “hexene” is acceptable when the double bond is at the first carbon.
- Compound #7 should be named “pentene,” not “butene,” reflecting the correct chain length.
- Isopentanone can be an acceptable name for certain ketones depending on substituents.
Summary of Key Points
- Double-check longest carbon chain length for precise base naming.
- Identify stereocenters and assign E/Z configurations for double bonds.
- Use commas and hyphens accurately in substituent numbering.
- Follow official IUPAC conventions rather than informal or shorthand names.
- Apply accepted simpler names where appropriate, such as “hexene” for terminal double bonds.
How do I correctly count the carbons in the longest chain?
Count the carbons carefully to ensure you select the longest continuous chain. Miscounting can change the molecule’s name and properties. Double-checking can help prevent errors.
When should I use E/Z designations for stereocenters?
Use E or Z when there is a double bond causing stereoisomerism. For example, if carbons 2 and 5 have double bonds with different groups attached, assign E or Z to indicate their configuration.
What is the correct way to write multiple substituent positions in a name?
Separate numbers for substituent positions with commas and link numbers to letters with hyphens. For instance, 3,5-diethyl is correct, while 3-5-ethyl is not.
Can I just name a compound like hexene without specifying the double bond position?
Yes, if the double bond is at the first carbon, it is often implied. Naming it simply as hexene is generally accepted in this case.
Is “isopentanone” an acceptable name for certain compounds?
Isopentanone can be used informally but may not always be official IUPAC nomenclature. Check context or ask if unsure to confirm.




Leave a Comment