Why Does Bromide Appear to Attack the Least Substituted Carbon?
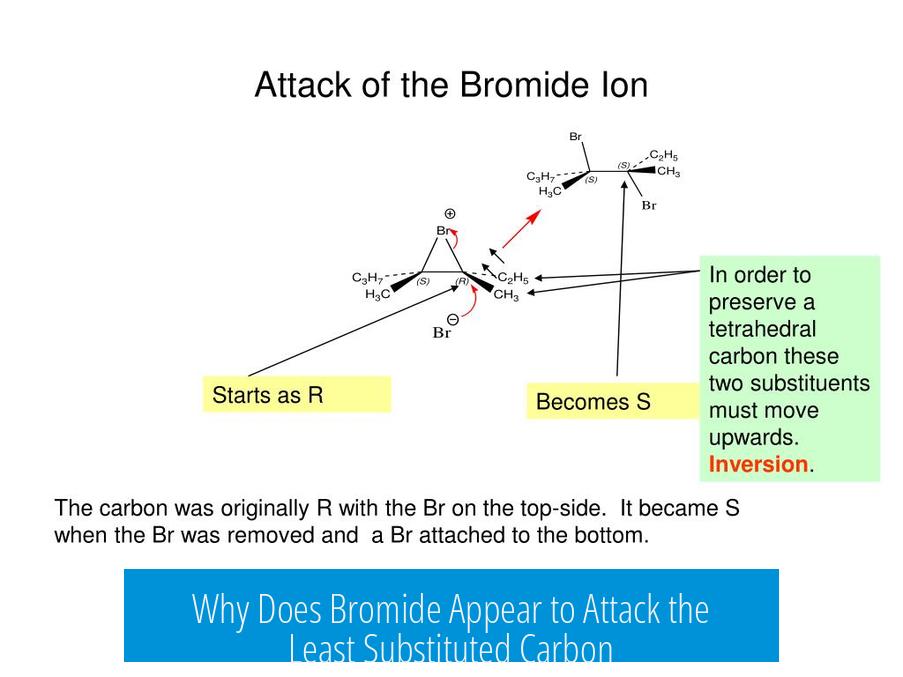
The apparent attack of bromide on the least substituted carbon of an alkene is a misinterpretation of the reaction mechanism. Actually, bromide does not nucleophilically attack the alkene at the less substituted carbon. Instead, the reaction proceeds through formation of a bromonium ion intermediate, which is opened by a nucleophile attacking the more substituted carbon. Bromide ends up bonded to the less substituted carbon as a consequence of this mechanism, not because it attacks there directly.
Understanding the Bromonium Ion Intermediate
The reaction of bromine with an alkene initially creates a bromonium ion. Bromine molecule (Br2) polarizes as one bromine acts as an electrophile (Br+), bonding simultaneously to both carbons of the alkene double bond. This forms a three-membered cyclic bromonium ion where the former double bond carbons share a positively charged bromine bridge.
Importantly, the positive charge in this intermediate is more stabilized on the more substituted carbon. This occurs because more substituted carbons better stabilize positive charge due to hyperconjugation and inductive effects. The bromonium intermediate thus has a partial positive charge skewed toward the more substituted carbon, making it more electrophilic and susceptible to nucleophilic attack there.
| Step | Description |
|---|---|
| Formation of bromonium ion | Br2 reacts with alkene; Br+ bridges both carbons |
| Charge stabilization | Positive charge localizes more on the more substituted carbon |
The True Nucleophilic Attack: Not by Bromide
Subsequent to bromonium ion formation, a nucleophile attacks to open the ring. This nucleophile can be bromide ion (Br-) or another nucleophile like methanol (CH3OH) if it is present in the reaction medium.
Crucially, the nucleophile attacks the more substituted carbon of the bromonium ion. The backside attack opens the three-membered ring in an anti addition fashion. This attack site matches where the positive charge is more stabilized, which makes the carbon more electrophilic and more reactive toward nucleophiles.
- More substituted carbon: Site of nucleophilic attack and ring opening
- Less substituted carbon: Retains the bond with the bromonium intermediate bromine
Why Does Bromide End Up on the Less Substituted Carbon?
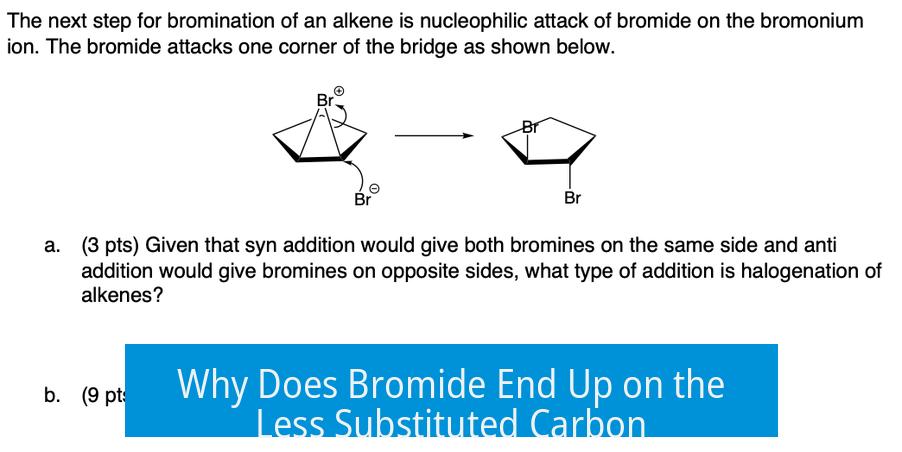
When bromide acts as the nucleophile, it attacks the more substituted carbon of the bromonium ion and opens the ring. Meanwhile, the positively charged bromine that bridges the carbons forms its covalent bond with the less substituted carbon. The bromide ion does not initiate the attack on the less substituted carbon; rather, it remains bound there after ring opening.
This leads to final products where bromine is attached to the less substituted carbon and the nucleophile to the more substituted carbon. This often appears as though bromide “attacks” the less substituted carbon, but mechanistically it is the bromonium intermediate bromine that stays there, not the attacking nucleophile.
Influence of Solvent and Nucleophile Concentration
The nature of the nucleophile and its concentration strongly affect which carbon the nucleophile attacks. For example, when methanol is the nucleophile and solvent, it attacks the more substituted carbon to open the bromonium ring because methanol is more abundant than bromide.
This alters regioselectivity toward Markovnikov addition, where the nucleophile adds to the more substituted carbon and bromide ends up on the less substituted one. When bromide ion predominates, it follows the same pathway but as nucleophile.
Anti Addition and Reaction Stereochemistry
The nucleophilic attack on the bromonium ion occurs through an SN2-type backside attack. This mechanism forces anti addition of substituents across the original double bond, resulting in trans stereochemistry of the final product.
- Backside nucleophilic attack opens three-membered bromonium ring.
- Anti stereochemistry is conserved in products.
- Nucleophile bonds to more substituted carbon; bromonium-bound bromine stays on less substituted carbon.
Summary of Key Points
- Bromonium ion formation: Br2 forms a three-membered ring with alkene carbons, with partial positive charge localized more on the more substituted carbon.
- Nucleophilic attack site: The nucleophile attacks the more substituted carbon due to greater carbocation stability.
- Bromide position: Bromide stays bonded to the less substituted carbon after ring opening; it does not nucleophilically attack that position.
- Anti addition mechanism: The ring opens via backside nucleophilic attack resulting in anti stereochemistry of the product.
- Solvent effects and nucleophile concentration: Influence whether bromide or another nucleophile attacks and determine regioselectivity accordingly.
Why does bromide appear to attack the least substituted carbon instead of the more substituted one?
The bromide ion does not actually attack the least substituted carbon. Instead, the bromonium intermediate forms with positive charge more stabilized on the more substituted carbon. The nucleophile attacks this carbon, opening the ring, so bromide ends up bonded to the less substituted carbon.
What role does the bromonium intermediate play in regioselectivity?
The bromonium intermediate is a three-membered ring where bromine bonds to both carbons of the double bond. Its positive charge is better stabilized on the more substituted carbon, guiding the nucleophile to attack there, not bromide.
Why does the nucleophile attack the more substituted carbon?
The more substituted carbon stabilizes the positive charge better. This makes it more electrophilic, attracting the nucleophile. Thus, the nucleophile attacks the more substituted carbon, breaking the bromonium ring.
How does solvent concentration affect the site of attack?
In solvents like methanol, which are in high concentration, the nucleophile mainly attacks the more substituted carbon. Bromide ions are present in lower amounts, so the nucleophile dominates the attack.
Why is the product regioselectivity anti-Markovnikov for bromide attachment?
Because the nucleophile attacks the more substituted carbon first, bromide ends up bonded to the less substituted carbon when the bromonium ring opens. This gives the appearance of bromide adding to the less substituted carbon, though it does not directly attack there.




Leave a Comment