Is the Tautomerization Step Correctly Represented?
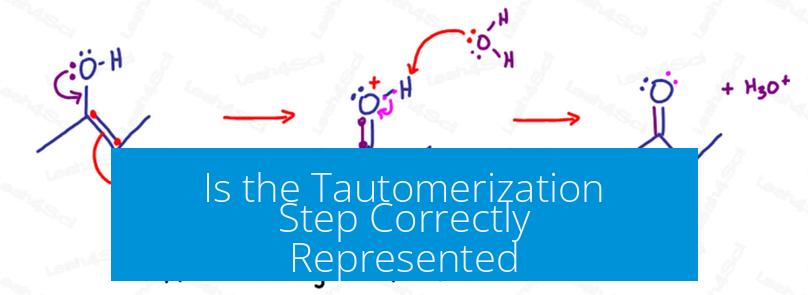
The tautomerization step is commonly misrepresented when shown as a concerted, intramolecular process without solvent involvement. Accurate depiction requires acknowledging key mechanistic and structural details. Typically, the process occurs through solvent-assisted, intermolecular proton transfers rather than through a single-step intramolecular rearrangement.
Nature of the Tautomerization Process
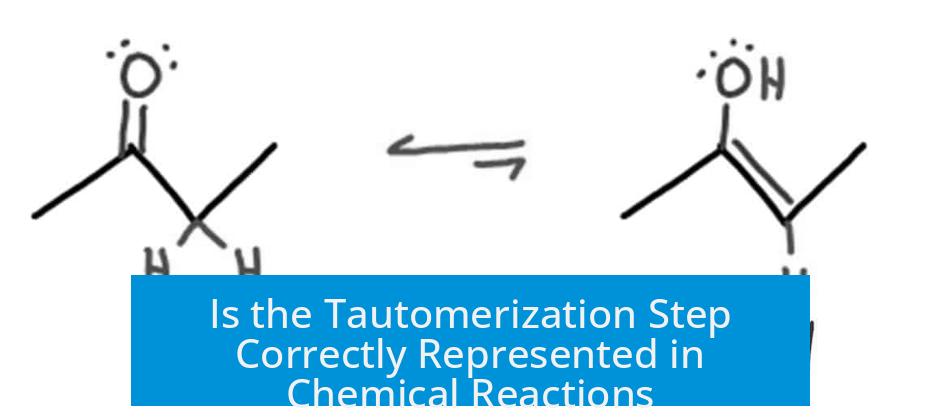
Tautomerization does not proceed via a concerted intramolecular pathway. Instead, it involves proton motions mediated by the surrounding solvent. The equilibrium between keto and enol forms involves either:
- Proton pickup at the alkene carbon by the enol, followed by deprotonation at the oxygen.
- Initial deprotonation of the oxygen, generating an enolate which then picks up a proton at the alkene carbon.
This solvent assistance makes tautomerization fundamentally an intermolecular process. Realistic representations illustrate interactions between at least two molecules, emphasizing proton shuttling via solvents or other species.
Evidence from astrochemical studies supports this model. Vinyl alcohol, the enol tautomer of acetaldehyde, is detectable in the low-pressure environment of space. This confirms that molecules do not readily tautomerize without intermolecular interactions.
Mechanistic Representation and Common Errors
Depicting tautomerization as a single-step reaction often introduces mistakes in arrow pushing. Reaction arrows must reflect electron flow, not atom or proton movement. Misuse of arrows to show direct hydrogen shifts confuses the mechanism.
Accurate mechanisms split the process into discrete steps involving proton transfer and bond reorganization. The proton moves via solvent or base-mediated pathways, not by direct migration within the molecule.
Structural and Bonding Considerations
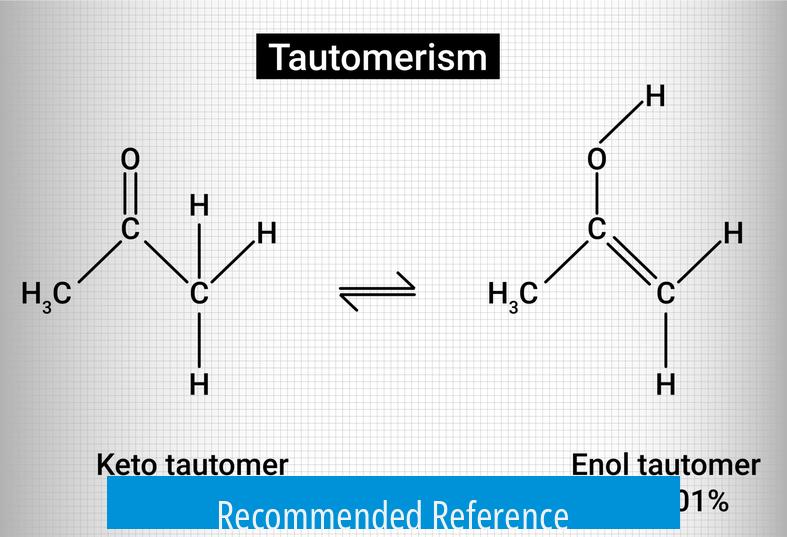
Tautomerism involves changes in key bonds. Typically, the enol form has a carbon-carbon double bond and a hydroxyl group, while the keto form presents a carbonyl double bond and single bonds appropriately rearranged. Representations must correctly show:
- Breaking and forming of double and single bonds
- Proper placement of hydrogen atoms consistent with proton transfers
Ignoring these details can misrepresent the chemical transformation and confuse students regarding electron and proton motions.
Recommended Reference
For proper mechanism representation and pedagogy, consult the “OREO” model of tautomerization:
https://pubs.acs.org/doi/abs/10.1021/ed100437b
This resource clarifies stepwise proton transfers, solvent involvement, and correct arrow pushing in tautomerization mechanisms.
Key Takeaways
- Tautomerization is a solvent-assisted, intermolecular process, not concerted intramolecular.
- Representations must show proton transfer via solvent or base, not direct intramolecular shifts.
- Reaction arrows should depict electron movement, not proton movement.
- Correct bond changes include switching double bonds from C=C in enol to C=O in keto forms.
- Use reliable references like the OREO mechanism for accurate understanding and representation.
Is tautomerization a concerted intramolecular process?
No, tautomerization is not a concerted intramolecular process. It involves multiple steps and usually requires the involvement of solvent molecules to assist proton transfers.
Should tautomerization be shown as involving one or multiple molecules?
Tautomerization is an intermolecular process. To represent it accurately, you need to show at least two molecules interacting, as proton transfers occur between them.
Are the common arrow-pushing mechanisms for tautomerization correct?
No, many common depictions show incorrect arrow pushing. Arrows must indicate electron movement, but some show proton movement instead, which is misleading and inaccurate.
What is the correct way to depict bond changes during tautomerization?
The mechanism must reflect changes in bonding, specifically the shift between double and single bonds and proton transfer on oxygen. The typical double bond–single bond–double bond sequence is key.
Where can I find a reliable reference for the correct tautomerization mechanism?
Refer to the OREO mechanism as detailed in this publication: https://pubs.acs.org/doi/abs/10.1021/ed100437b. It offers an accurate stepwise explanation with proper electron flow.



Leave a Comment