Understanding the Dehydration Reaction: Why A is the Correct Answer
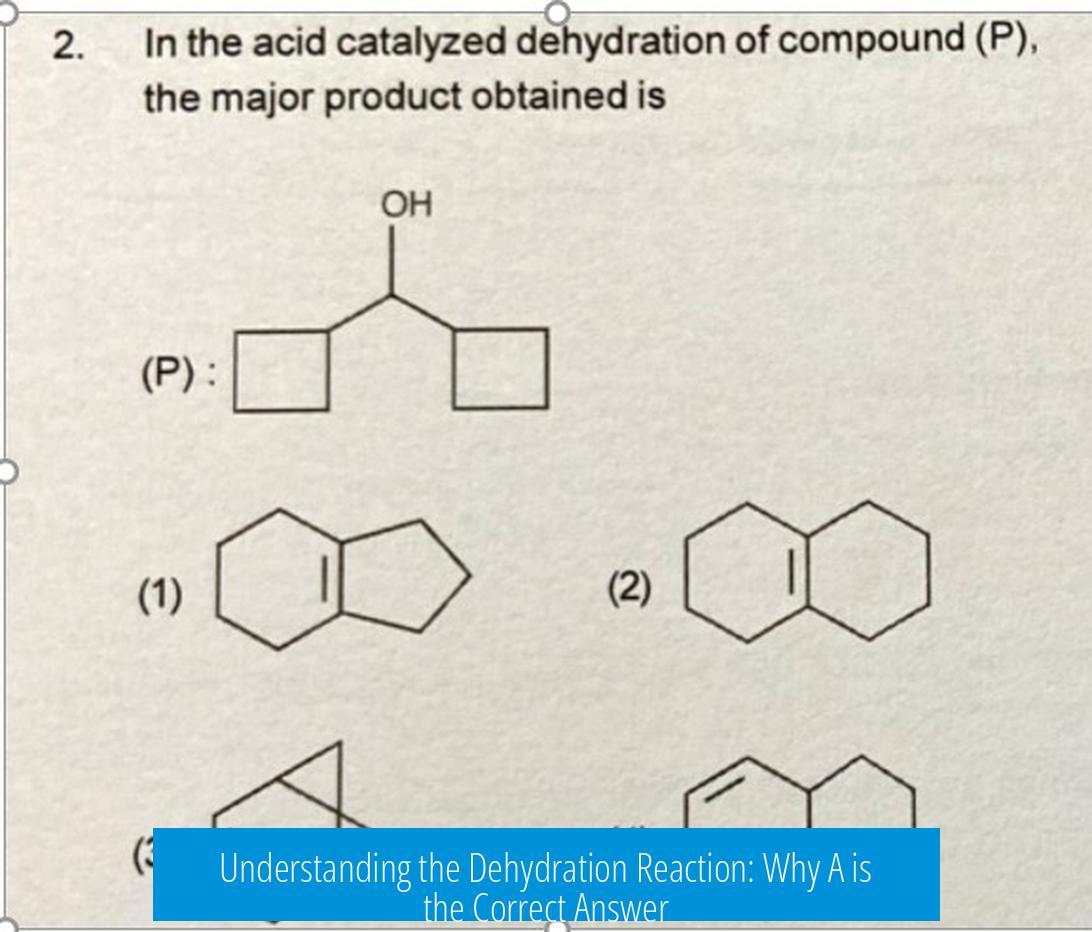
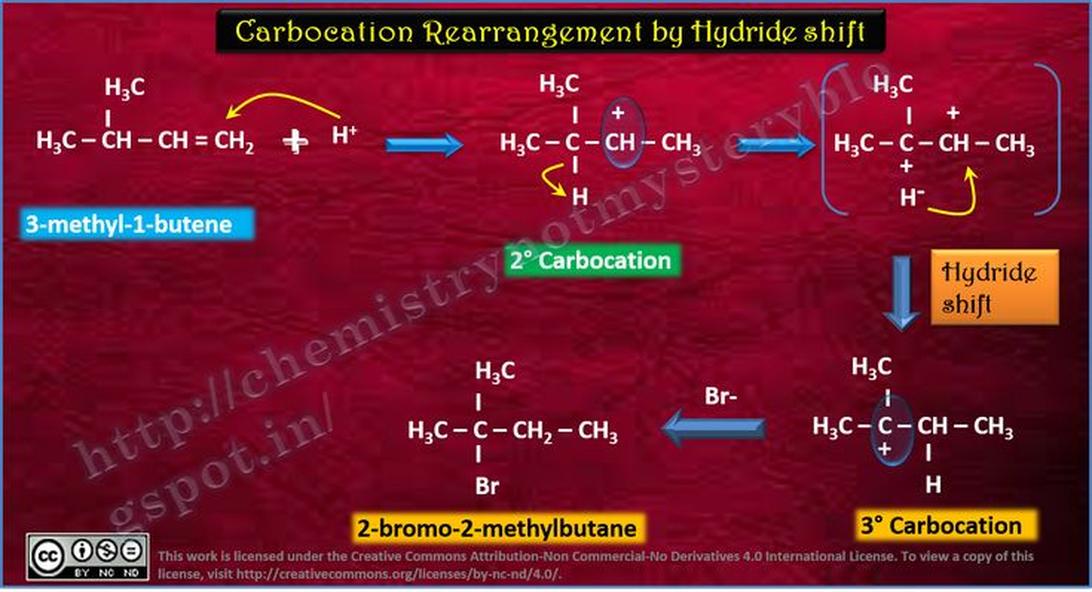
The correct answer is A. This reflects the major thermodynamic product in the dehydration of the alcohol, formed after carbocation rearrangement via a 1,2 methyl shift. This intermediate leads to a more stable tertiary carbocation and ultimately a more substituted, stable alkene.
Mechanism of Dehydration and Carbocation Formation
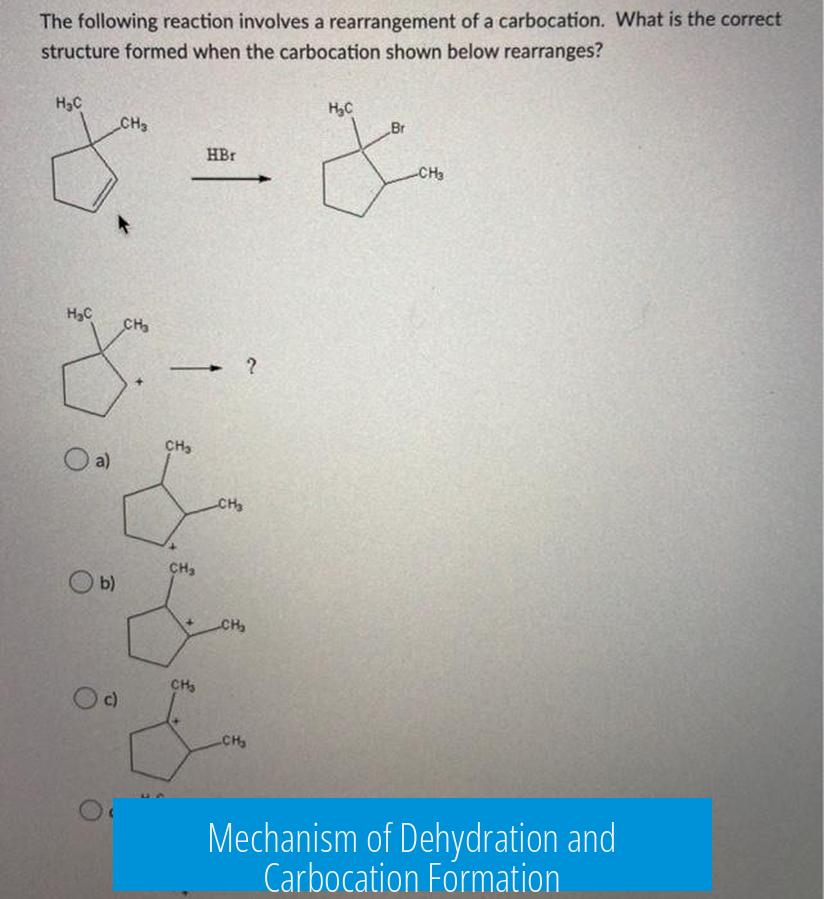
The dehydration of an alcohol under acidic conditions proceeds through an E1 mechanism. This involves protonation of the hydroxyl group to form water—a good leaving group—followed by loss of water and formation of a carbocation. The carbocation intermediate is key to understanding the product distribution.
This carbocation can rearrange through shifts to increase its stability. Two common shifts are:
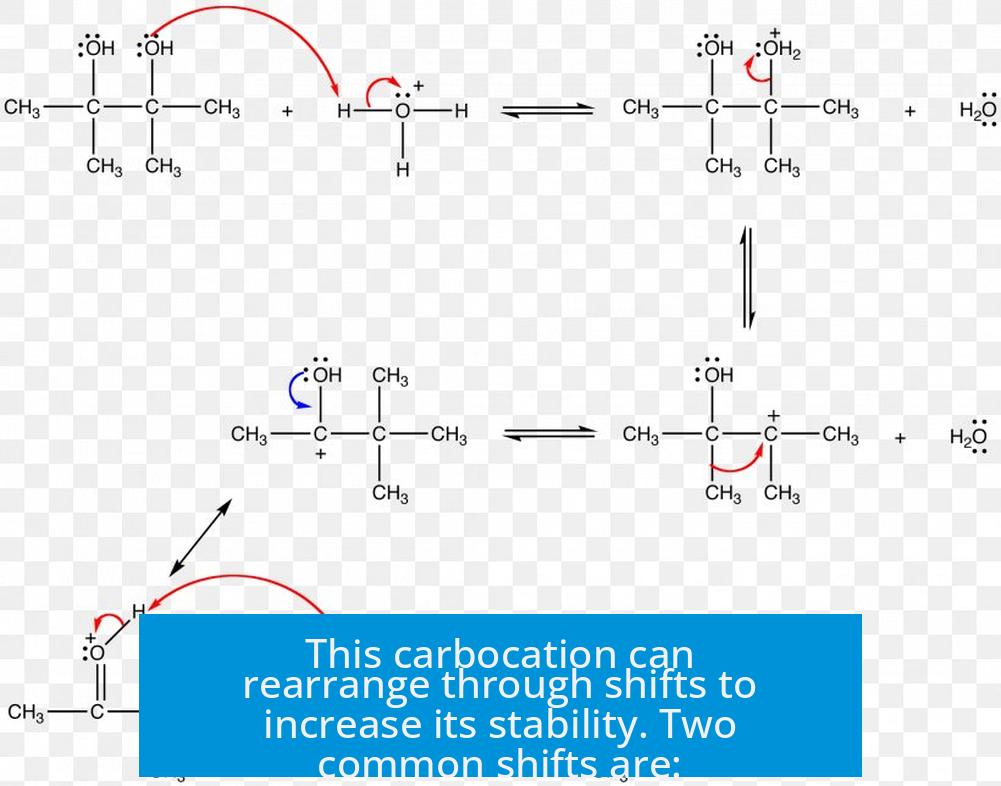
- 1,2 Methyl shift: A neighboring methyl group migrates to the carbocation center.
- 1,2 Hydride shift: A hydride (hydrogen with its pair of electrons) moves to the carbocation.
In this case, the 1,2 methyl shift is favored over hydride shifts due to the greater stability of the resulting tertiary carbocation compared to a secondary one.
Why the Methyl Shift Leads to Answer A
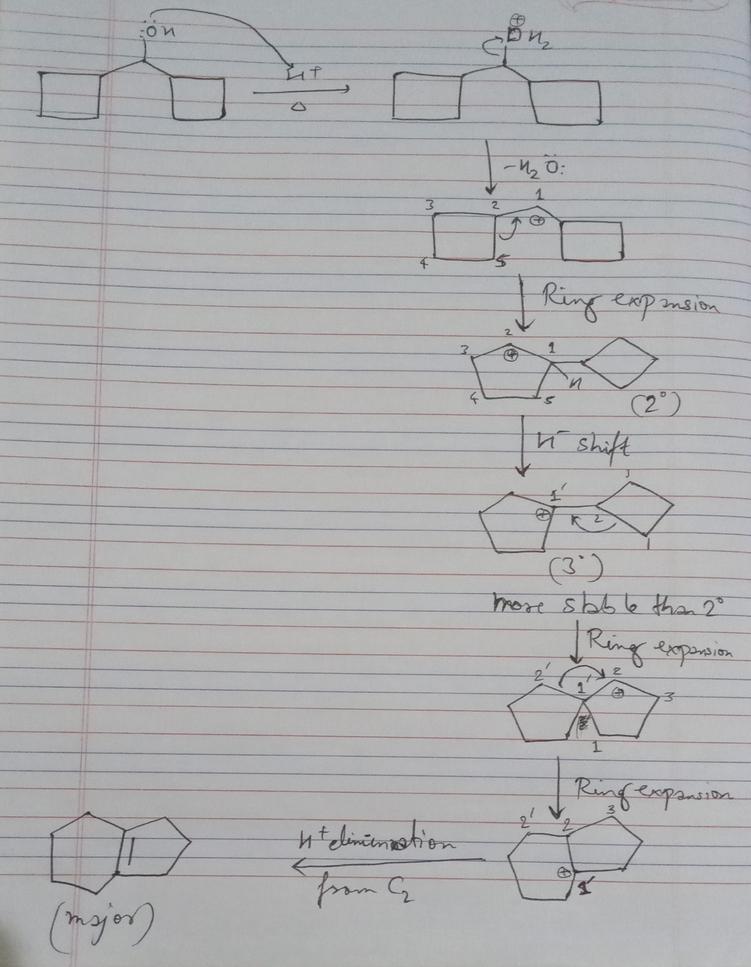
Answer A corresponds to the alkene formed after the methyl shift. This shift moves the carbocation to a more substituted carbon, increasing its stability. The more stable carbocation then loses a proton to form the most stable, most substituted alkene.
- This alkene is thermodynamically favored due to substitution stabilization.
- The conditions—concentrated acid and heat—favor equilibrium, allowing rearranged carbocations to form.
- Ultimately, the reaction favors the most stable product rather than the fastest to form.
Kinetic vs Thermodynamic Products: Explaining Your Friend’s Choice of C
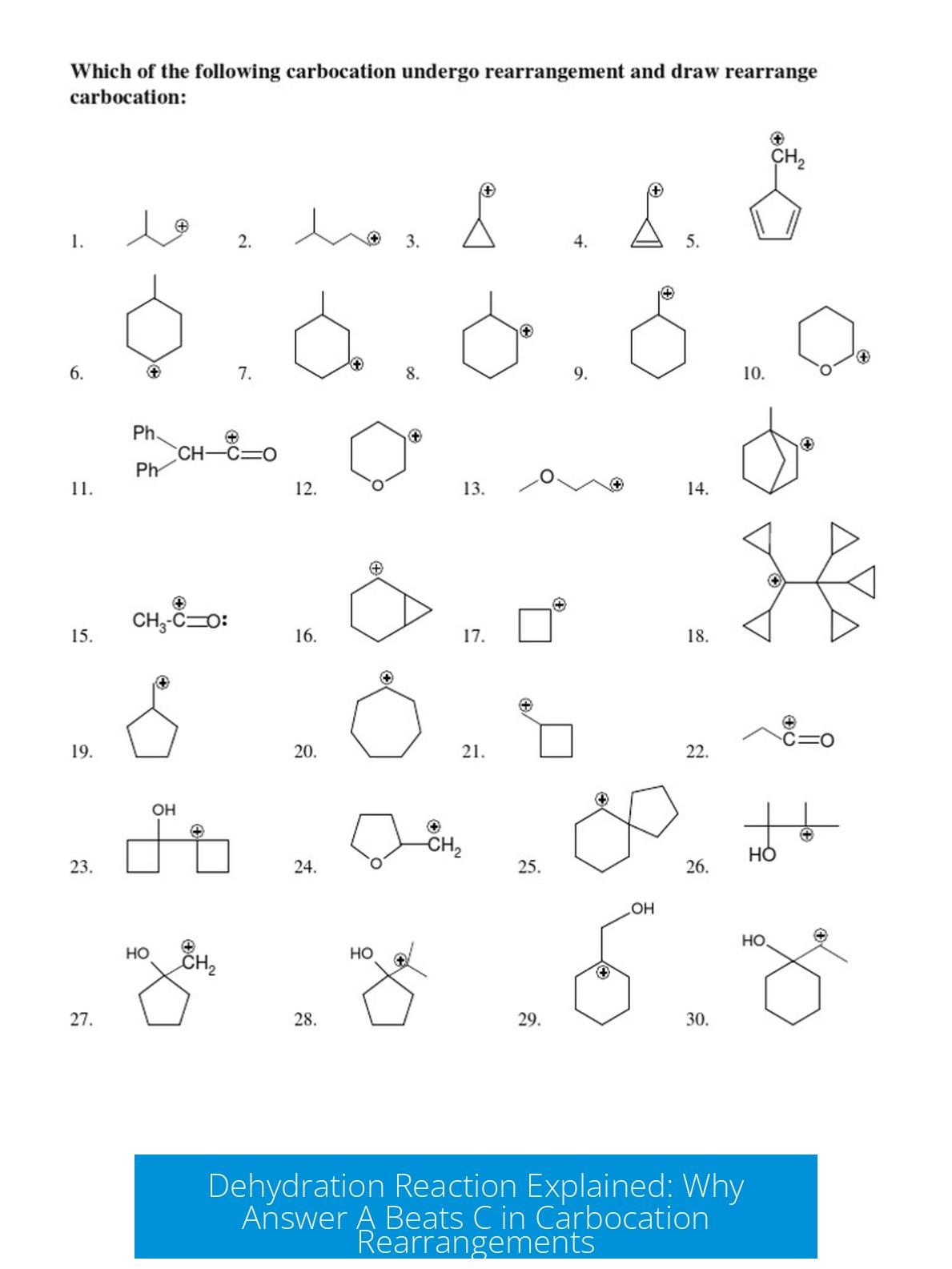
Your friend’s choice, answer C, likely reflects the kinetic product. This is formed directly from the initial carbocation without rearrangement. It corresponds to E1 elimination where no carbocation shifts occur.
Under lower temperature or shorter reaction time, this kinetic product might predominate because it has a lower activation energy pathway. However, this product is less substituted and less stable.
Effect of Reaction Conditions
The reaction environment strongly influences which product dominates:
| Condition | Predominant Product | Reason |
|---|---|---|
| Concentrated acid, heat, long time | A (thermodynamic product) | Allows carbocation rearrangement; reversibility drives reaction toward most stable alkene. |
| Lower temperature, short time | C (kinetic product) | Faster formation with no carbocation shift; less stable but easier to form initially. |
Summary of Key Evidence Supporting Answer A
- Methyl shifts offer favored routes to stabilize carbocations over hydride shifts.
- The tertiary carbocation formed after the methyl shift leads to highly substituted, stable alkenes.
- The reaction is under thermodynamic control due to heat and acid, favoring rearrangement and stability.
- Experimental and theoretical insights confirm that the major product after equilibrium is answer A.
- Kinetic product C can appear transiently, but it converts to thermodynamic product A over time.
Additional Points on Acid Choice and Mechanism
Sulfuric acid (H2SO4) provides a strong, concentrated proton source. This protonates the hydroxyl group, enabling its departure and forming the carbocation intermediate.
Compared to weaker or different acids, sulfuric acid ensures efficient protonation and facilitates elimination via E1. This environment favors rearrangements and formation of the most stable product.
—
Key Takeaways
- The major product under typical dehydration conditions is A, formed via a methyl shift leading to a tertiary carbocation.
- Kinetic product C forms quickly without rearrangement but is less stable and converts to A over time.
- Rearrangements such as methyl shifts stabilize carbocations, influencing product distribution.
- Thermodynamic control under heat and acid drives formation of the most substituted alkene (answer A).
- Careful analysis of reaction conditions and mechanisms supports A as the correct choice.
Decoding the Mystery: Why Answer A Triumphs Over C in Carbocation Rearrangements
Ah, the age-old chemistry debate: you say A, your friend counters with C. Which side wins? Let’s unravel this puzzle with facts, logic, and a pinch of chemistry wit, shall we?
First things first: the correct answer here is A. Why? Because it aligns perfectly with the well-established mechanism of carbocation formation and rearrangement during dehydration reactions. It’s like choosing the best path on a treasure map—A takes you straight to the gold.
Why Does Carbocation Rearrangement Lead to Answer A?
When an alcohol loses water in an elimination reaction, it often forms an intermediate carbocation. This carbocation is a bit like a restless traveler looking for a more comfortable spot. It doesn’t stay put; it rearranges to a more stable position if possible.
Here’s the kicker: this rearrangement can happen through shifts of hydride ions (hydrogen with its electron) or methyl groups (a CH3 group). Among these, the methyl shift is usually the favored route.
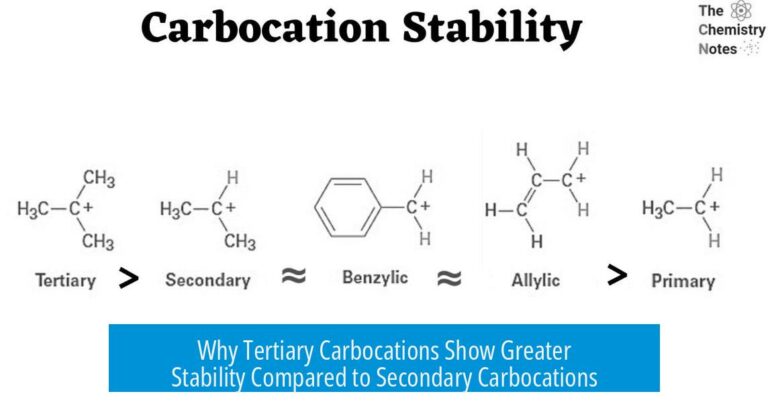
- A methyl shift often leads to a tertiary carbocation, which is more stable due to better electron donation compared to a less substituted carbocation.
- This stability translates to a major formation of product A, where the double bond ends up in the most substituted—and thus most stable—position.
Meanwhile, your friend’s answer C corresponds to the product formed by E1 elimination without any rearrangement. This product does form but is usually a minor player, akin to the understudy actor who briefly steps on stage before the star steals the show.
Thermodynamics vs Kinetics: The Drama Behind Product Formation
Here’s a chemistry classic: thermodynamic products vs. kinetic products. Think of it like choosing between the fastest route home or the scenic, safest highway.
The kinetic product (answer C) forms quickly because it requires less energy—no rearrangements needed. But it’s less stable.
On the other hand, the thermodynamic product (answer A) might take longer to form since it involves the methyl shift rearrangement, which has a higher activation barrier. However, it is more stable in the long run.
Since the reaction conditions involve concentrated acid and heat (hello, ample energy!), the system favors the thermodynamic product. Over time and with heat, the reaction mixture shifts more and more toward A, making it the major product observed.
Real Lab Conditions: Expect a Mixture
Reality check: in a real lab, you’d get a bit of everything—a blend of products 1, 2, and 3 (in this case A, the minor others, and C). Chemistry rarely plays by pure theory when chemicals get involved.
But if you let the reaction stir and heat long enough, the magic of reversibility and equilibration pushes the balance toward product A, confirming its dominance.
What About That Methyl Shift? Why Is It the MVP?
A question worth asking! Why does a methyl shift win over a hydride shift?
The simple explanation is the principle of migratory aptitude. Both methyl and hydride shifts can occur, but the methyl group has a higher tendency to migrate when it leads to a more substituted (and thus more stable) carbocation.
In this scenario, the methyl shift allows the formation of a tertiary carbocation, a sweet spot of stability in carbocation chemistry. The hydride shift sometimes happens but is less favored because moving the methyl group better stabilizes the system.
Reaction Conditions Seal the Deal
Reactions aren’t just about molecules dancing in isolation; the environment matters.
This dehydration takes place in concentrated sulfuric acid and heat. The acid protonates the alcohol, facilitating water loss and carbocation formation. Heat provides the energy to overcome activation barriers, like getting over a hill on a bike rather than just coasting downhill.
This combination favors the reversible and thermodynamic pathway, meaning the system can “fix” initial products by rearrangement until it settles on the most stable product, answer A.
Summing Up: Why You’re Right and Your Friend Isn’t Wrong… Exactly
- You’re right: Product A, formed after a 1,2 methyl shift, is the major, most stable product favored under these reaction conditions.
- Your friend isn’t totally wrong: Product C, the kinetic product, does form initially but usually converts to A over time with heat.
- This scenario highlights the importance of understanding both kinetics and thermodynamics to properly predict reaction outcomes.
- The key is the carbocation rearrangement stabilizing the intermediate and driving the formation of A.
What Can You Take Away From This?
If you ever get stuck deciding between two answers in organic chemistry, ask yourself:
- Which product is more substituted or stable?
- Are reaction conditions favoring kinetic or thermodynamic control?
- Is there a carbocation intermediate that might rearrange?
- How long is the reaction run, and at what temperature?
Applying this approach can clear up many confusing cases.
So next time the chemistry discussion heats up—literally—remember this case: answer A stands as the champion because it represents the thermodynamic masterpiece sculpted by carbocation rearrangement and reaction conditions. And your friend’s C? Well, it’s a worthy contender, just not the final winner here.
Science is less about being right instantly and more about understanding the journey of how things reach their most stable state.
Need More Help? Let’s Talk Shift Mechanisms!
Curious about how methyl and hydride shifts work? Want to see step-by-step diagrams? Or maybe you want to explore other reactions where kinetics take the lead? Just ask, and we’ll dive deep—with no mystery left unsolved!
Why is answer A considered correct over C in this reaction?
Answer A forms after a methyl shift rearrangement, creating a more stable tertiary carbocation. This leads to a more substituted and stable alkene. Answer C represents a direct elimination without rearrangement, which is less stable and usually the kinetic product.
Can the product C still form during the reaction?
Yes, C can form initially as the kinetic product. But under heated acidic conditions, the reaction favors the thermodynamic product A. Over time, C can rearrange or convert into A due to reversibility of elimination.
How do reaction conditions affect whether product A or C forms?
Concentrated acid and heat promote carbocation formation and rearrangement. This favors the most substituted and stable alkene, product A. Lower temperatures or short reaction times can lead to the kinetic product C instead.
What is the role of methyl and hydride shifts in deciding the product?
Methyl shifts are favored when they lead to a more stable carbocation. In this case, the methyl shift creates a tertiary carbocation, stabilizing it. Hydride shifts are less favored here. This is why product A is more favored.
Why might someone think the answer is C initially?
Product C forms by simple E1 elimination without rearrangement, which is faster and occurs early. If the reaction is stopped early or under mild conditions, C might predominate. This can cause confusion if the reaction is considered at kinetic control.



Leave a Comment