Understanding Retrosynthesis: An Essential Tool in Organic Chemistry
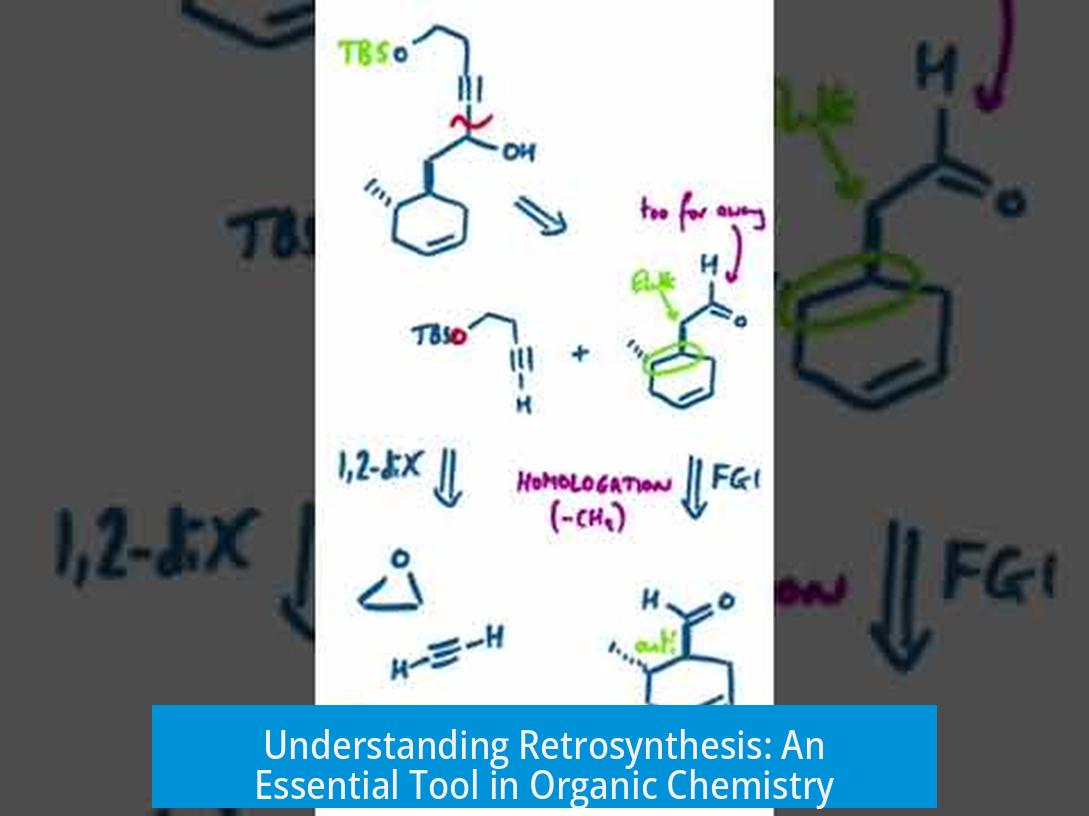
Retrosynthesis is a strategic approach to organic synthesis where chemists start with the desired final product and work backwards to simpler, commercially available precursors or materials. This method guides chemists through complex synthetic routes by breaking down target molecules into manageable building blocks. It becomes especially valuable in planning multistep syntheses of complex structures, such as natural products or pharmaceuticals.
Why Retrosynthesis Matters
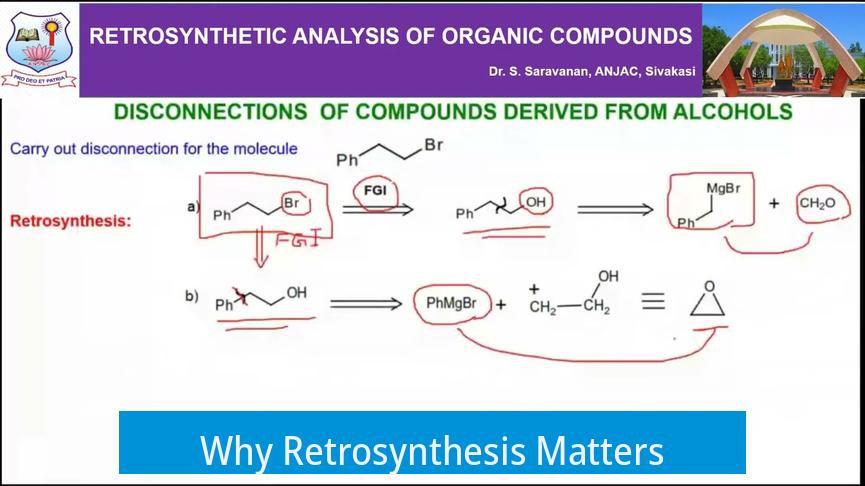
Retrosynthesis transforms the synthesis process from trial and error into a structured plan. The important aspect is that chemists usually begin with a clear target in mind but no fixed starting material. By starting at the product and reversing the synthesis steps, chemists reveal multiple possible routes to reach that molecule. Some routes offer higher yields, better practicality, or simpler intermediates.
- It helps identify feasible approaches and alternatives that forward synthesis alone might miss.
- It reveals intermediates that are easier to obtain or synthesize from commercially available substances.
- It reduces reliance on trial-and-error for complex, bioactive molecules; instead, synthesis is methodically designed.
With retrosynthesis, chemists simplify complexity by dissecting molecules systematically. The backward approach ensures that the synthesis moves from complex targets to simple components rather than attempting to assemble complex molecules blindly.
Retrosynthesis Compared to Forward Synthesis
Forward synthesis starts with accessible reagents and attempts to build the target molecule step by step. Retrosynthesis does the opposite by starting at the target and peeling it apart into known fragments.
For single-step reactions, retrosynthesis offers minimal advantage. Yet for multistep syntheses—typically involving four or five or more stages—it becomes invaluable.
Some key benefits over working forward include:
- Identification of crucial intermediates and connections that can simplify the route
- Recognition of incompatible functional groups and planning around them
- Optimization of reaction order to prevent premature deactivation of intermediates
Sometimes solving synthetic routes requires thinking both forwards and backwards. Retrosynthesis enhances this problem-solving skill, allowing chemists to test multiple potential disconnections for efficiency or feasibility.
Complexity and the Role of Retrosynthesis
Retrosynthesis becomes particularly essential when dealing with complex molecules with multiple functional groups or stereocenters. Examples include natural products like Taxol, where infinite synthetic possibilities exist.
In undergraduate organic chemistry, students often manage simple reactions without retrosynthetic planning because the reaction toolbox is limited. However, once synthesis complexity grows or starting materials are not predefined, retrosynthesis emerges as a critical strategy.
Unlike multistep synthesis, where one practical answer usually exists, retrosynthesis offers multiple valid pathways. The challenge lies in selecting the most practical, efficient, or high-yield route.
Approach and Mindset in Retrosynthesis
The core mindset for retrosynthesis asks: “Which bonds in the target molecule do I know how to form?” This transforms organic synthesis from a guessing game into a rational design process applicable to any molecule.
Viewing retrosynthesis as a structured puzzle, rather than trial-and-error progression, prepares chemists for increasingly challenging targets where simple forward logic fails.
Adopting this perspective is critical at advanced levels of organic synthesis, particularly when designing routes for complex natural or bioactive compounds.
Learning and Practicing Retrosynthesis
Developing retrosynthesis skills requires practice. Repeatedly analyzing synthesis problems helps recognize common “disconnections” and patterns.
- Organic chemistry textbooks often include synthesis problems in chapters after basic reactions are introduced.
- Reading key works such as E.J. Corey’s “Disconnection Approach”—considered the bible of organic synthesis—enhances understanding.
- Diving into synthesis problems that challenge the mind to break molecules into precursor parts builds intuition.
Practice also reveals frequently used retrosynthetic “moves” or strategic bond disconnections, which become familiar over time.
Practical Examples Illustrating Retrosynthesis
Retrosynthesis’s importance becomes clear in specific reactions.
- For electrophilic aromatic substitution (EAS) and nucleophilic aromatic substitution (SnAr), the order of reactions affects product reactivity.
- Performing these in the wrong sequence can drastically reduce intermediate reactivity, hindering subsequent steps.
Planning steps backward from the desired product helps avoid such pitfalls by anticipating functional group compatibility throughout the synthetic sequence.
Exercises to Enhance Retrosynthesis Skills
Several approaches encourage skill development:
- Attempt retrosynthesis on complex natural products, especially those without a known total synthesis.
- Compare strategies to synthesize simple terpenes or other molecules both with and without retrosynthetic reasoning.
- Practice identifying bond disconnections relevant to target molecules and connecting them to known reactions.
This hands-on approach fosters problem-solving abilities and prepares chemists to address diverse synthetic challenges.
Summary of Key Points
- Retrosynthesis is a backward planning method beginning at the target molecule and progressing to simpler precursors.
- It becomes indispensable for multistep syntheses, especially with complex molecules.
- Retrosynthesis provides multiple synthetic routes and reveals useful intermediates not obvious with forward synthesis.
- The method improves functional group compatibility planning and step sequencing.
- Skill development requires practice and study of disconnection analysis, as popularized by E.J. Corey.
- Retrosynthesis refines synthetic design, making the complex rational and approachable.
What is the main advantage of retrosynthesis compared to forward synthesis?
Retrosynthesis starts from the final product and works backwards to simpler precursors. This approach helps identify multiple routes, including easier or more feasible ones, which might be missed by forward synthesis.
When does retrosynthesis become essential in synthesis planning?
It becomes critical for complex molecules with multiple steps, usually four or more. Simple molecules or single-step reactions rarely need retrosynthesis, but complex targets require backward analysis to handle many possibilities.
How does retrosynthesis help with choosing starting materials?
It allows chemists to work back to commercially available or easily prepared materials. Instead of guessing forwards, you begin with the target and find accessible intermediates that simplify the overall synthesis.
Can retrosynthesis be used for all types of molecules?
Yes, retrosynthesis is a universal method. It involves breaking down any target molecule into bonds you know how to form, making it a flexible strategy for natural products, bioactive compounds, or simpler molecules.
What mindset is necessary for successful retrosynthesis?
Thinking about which bonds to break and how to form them is key. Treat it as a problem-solving process, not just sequential steps. This mindset helps find solutions when straightforward synthesis plans fail.
How can one improve retrosynthesis skills?
Practice is crucial. Solve many retrosynthetic problems and study classical methods like Corey’s disconnection approach. Working through synthesis challenges regularly sharpens recognition of common patterns and moves.




Leave a Comment