Atoms are the building blocks of matter and the basic unit of any element. In an atom, there are three important subatomic particles: electrons, protons, and neutrons. The number of protons in an atom determines the element’s identity, the atomic number (Z). The number of protons, neutrons, and electrons in an atom also determine the element’s mass number (A).
Have you ever wondered what element has 10 electrons, 8 protons, and 10 neutrons? This is an important question in chemistry, as it can help us understand the structure of different elements. In this article, we will discuss the answer to this question and explore some of the related topics in more detail.
We’ll start with the basics. Every element has a unique atomic number (Z) which corresponds to the number of protons in the element’s atoms. For example, hydrogen has one proton, helium has two protons, lithium has three protons, and so on. Knowing the number of protons in an element allows us to distinguish it from all other elements.
We can also use the atomic number to determine the number of electrons in a neutral atom of that element. Neutral atoms have one electron for every proton, so if an element has eight protons, it will also have eight electrons. Similarly, if an element has 10 protons, it will have 10 electrons.
Now, let’s move on to neutrons. The number of neutrons in an atom is important in determining its mass number (A). The mass number is the total number of protons and neutrons in an atom. It can be calculated by adding the number of protons and the number of neutrons in the nucleus.
So, what element has 10 electrons, 8 protons, and 10 neutrons? The answer is oxygen. Oxygen is the element with an atomic number of 8 and a mass number of 18, meaning it has 8 protons and 10 neutrons. The number of electrons in a neutral atom of oxygen is 8, as there is one electron for every proton.
Knowing the number of protons, neutrons, and electrons in an atom is important for understanding the structure and composition of different elements. This knowledge can be used for a variety of scientific and industrial applications. It can also help us better understand the behavior of atoms, molecules, and compounds.
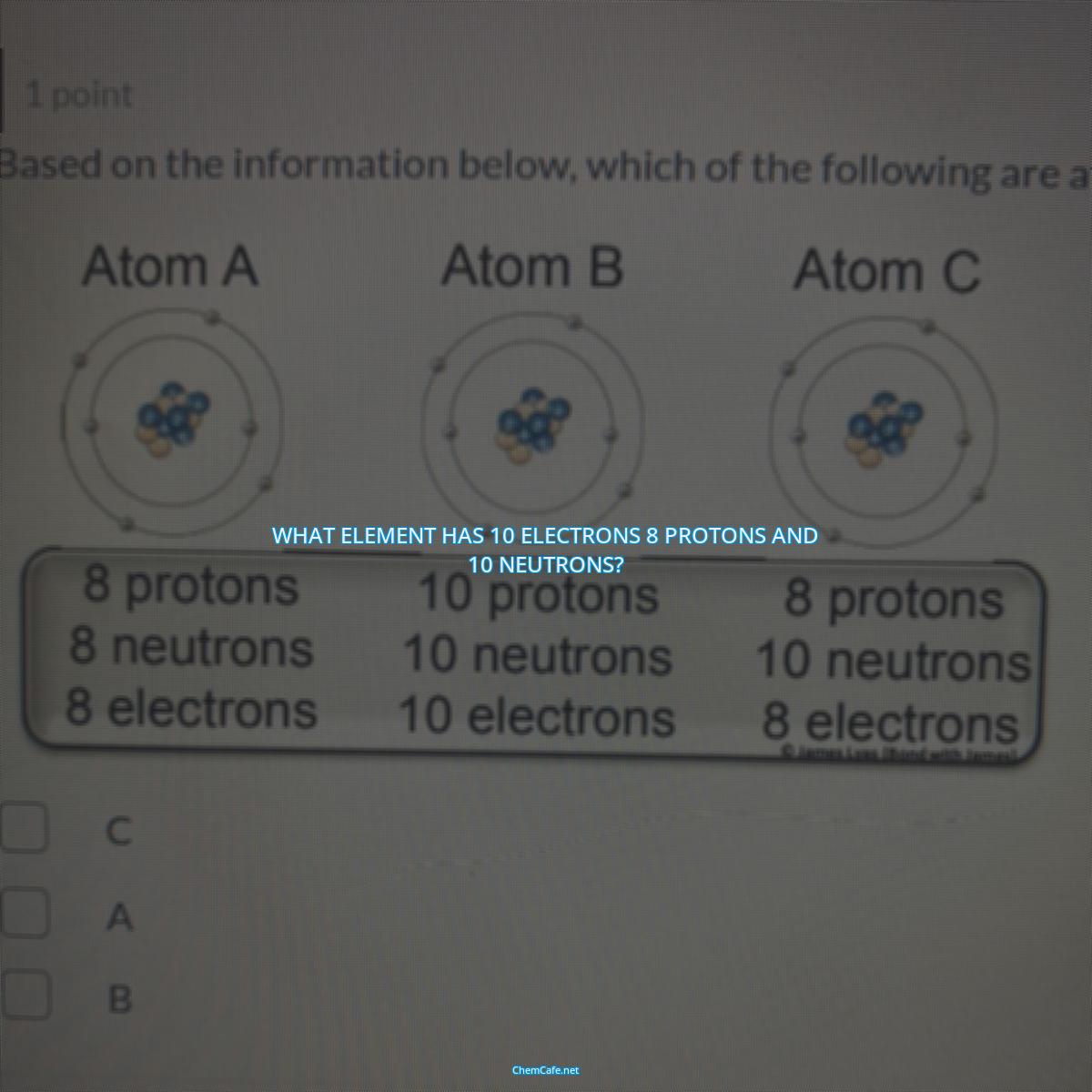
What element has 10 electrons 8 protons and 10 neutrons?
Atoms are made up of protons, neutrons, and electrons. Each element has a unique combination of these particles that gives it its own unique properties. While some elements have more protons than others, they all have the same number of electrons and neutrons.
The element with 10 electrons, 8 protons, and 10 neutrons is oxygen. Oxygen is a key component of the Earth’s atmosphere, and it is essential for the survival of most life forms. It is also a major part of the air we breathe and the water we drink.
Atomic Number and Mass Number of Elements
The number of protons in an atom is called its atomic number ((Z)). This number is very important because it is unique for atoms of a given element. All atoms of an element have the same number of protons, and every element has a different number of protons in its atoms. For example, all helium atoms have two protons, and no other elements have atoms with two protons.
The total number of protons and neutrons in an atom is called the mass number ((A)). Table (PageIndex{1}) shows the atomic numbers and mass numbers for the first six elements in the periodic table.
Table (PageIndex{1}): Atoms of the First Six Elements
| Element | Protons | Neutrons | Electrons | Atomic Number (Z) | Mass Number (A) |
|---|---|---|---|---|---|
| Hydrogen | 1 | 0 | 1 | 1 | 1 |
| Helium | 2 | 2 | 2 | 2 | 4 |
| Lithium | 3 | 4 | 3 | 3 | 7 |
| Beryllium | 4 | 5 | 4 | 4 | 9 |
| Boron | 5 | 6 | 5 | 5 | 11 |
| Carbon | 6 | 6 | 6 | 6 | 12 |
Of course, since neutral atoms have to have one electron for every proton, an element’s atomic number also tells you how many electrons are in a neutral atom of that element. An atom with three protons is a lithium atom, an atom with five protons is a boron atom, an atom with six protons is a carbon atom . . . the list goes on. Since an atom of one element can be distinguished from an atom of another element by the number of protons in its nucleus, scientists are always interested in this number, and how this number differs between different elements.
Summary
In summary, the element with 10 electrons, 8 protons, and 10 neutrons is oxygen. The atomic number of oxygen is 8, and its mass number is 16. This means that oxygen atoms have 8 protons and 8 electrons, and they have 8 neutrons. Oxygen is a key component of the Earth’s atmosphere, and it is essential for the survival of most life forms.
What has 8 protons and 10 electrons?
Atoms of elements are made up of protons, neutrons, and electrons. The number of protons in an atom is called its atomic number ((Z)). This number is very important because it is unique for atoms of a given element. All atoms of an element have the same number of protons, and every element has a different number of protons in its atoms. For example, all helium atoms have two protons, and no other elements have atoms with two protons.
The number of electrons in an atom can vary, but it must be equal to the number of protons in order for the atom to be electrically neutral. Atoms can gain or lose electrons, forming ions with a net positive or negative charge. Ions with a net negative charge are called anions, and ions with a net positive charge are called cations.
So, what has 8 protons and 10 electrons? The answer is the oxygen ion O²⁻. Oxygen is a non-metal element with atomic number 8, meaning that all oxygen atoms have 8 protons. Oxygen can form ions with a charge of either -2 or +2. In the case of the O²⁻ ion, two electrons have been removed from the atom, leaving 8 protons and 10 electrons. Therefore the number of protons in the O²⁻ ion is 8.
Net Charge of O²⁻ Ion
The net charge of an ion is the number of protons minus the number of electrons. In the case of the O²⁻ ion, the net charge is 8 – 10 = -2. This means that the O²⁻ ion has a net negative charge of -2. This charge is written as a superscript in the upper right corner relative to the symbol for oxygen atoms, so the O²⁻ ion is written as #O^2-# (the – sign should be part of the superscript).
Based on the above discussion, it is concluded that the O²⁻ ion has 8 protons and 10 electrons. Therefore option A is the correct answer. With eight protons, we are necessarily looking at oxygen, but with 10 electrons, the net charge on the ion is minus two. This charge is written as a superscript in the upper right corner relative to the symbol for oxygen atoms, so the O²⁻ ion is written as #O^2-# (the – sign should be part of the superscript).
What element has 8 protons 10 neutrons and 10 electrons?
Atoms are the building blocks of all matter, and each atom is made up of protons, neutrons, and electrons. Each element has a unique number of protons, and it is this number that determines the element’s identity. Therefore, when we are asked which element has 8 protons, 10 neutrons, and 10 electrons, the answer is oxygen.
Understanding Atoms and their Components
Atoms are made up of three particles, protons, neutrons, and electrons. Protons have a positive charge and are found in the nucleus of the atom. Neutrons have no charge and are also found in the nucleus. Electrons are found in shells surrounding the nucleus and have a negative charge.
The number of protons in an atom determines its atomic number ((Z)). The atomic number is also the same as the element’s position on the periodic table. The number of protons plus the number of neutrons is the atom’s mass number ((A)).
Table (PageIndex{1}) shows the number of protons, neutrons, and electrons for the first six elements. As you can see, the number of protons is the same for all atoms of an element, and each element has a different number of protons.
Oxygen Atoms
Oxygen is the eighth element on the periodic table, so its atomic number is 8. This means that all oxygen atoms have 8 protons. Since neutral atoms must have an equal number of protons and electrons, oxygen atoms also have 8 electrons.
To determine the number of neutrons in an oxygen atom, we use the mass number ((A)). The mass number of oxygen is 16, which means that oxygen atoms have 8 protons (from the atomic number) and 16 – 8 = 8 neutrons. Therefore, oxygen atoms have 8 protons, 8 neutrons, and 8 electrons.
In conclusion, the element with 8 protons, 10 neutrons, and 10 electrons is oxygen. It has an atomic number of 8 and a mass number of 16. Oxygen is the eighth element on the periodic table and is essential for life on Earth. Understanding the components of atoms is essential for understanding chemistry and the world around us.
What ion has 8 protons 8 neutrons and 8 electrons?
Atoms of an element are made up of protons, neutrons and electrons. The number of protons an atom has is its atomic number (Z), while the number of protons and neutrons together is its mass number (A). When an atom has more or fewer electrons than protons, it becomes an ion with a net positive or negative charge. So, what ion has 8 protons, 8 neutrons and 8 electrons?
Oxygen (O) – The Answer
In a neutral atom of an element, the number of electrons is equal to the number of protons. In addition, the atomic number of an element is the same as the number of protons. Therefore, with eight protons, we are necessarily looking at oxygen, but with 10 electrons, the net charge on the ion is minus two. This charge is written as a superscript in the upper right corner relative to the symbol for oxygen atoms; closest I can get to the write the symbol in this answer is O^2- (the – sign should be part of the superscript).
Understanding Protons, Neutrons, and Electrons
Protons and neutrons are located in the nucleus at the center of the atom, while electrons move around the nucleus in shells. Neutrons are neutral particles and have no charge, whereas electrons have a negative charge. It is the number of protons and electrons in an atom that determines its charge.
Atoms of the First Six Elements
To better understand the answer to the question, it is important to look at the atoms of the first six elements. The table below shows the number of protons, neutrons, and electrons in the neutral atoms of the first six elements.
| Name | Protons | Neutrons | Electrons | Atomic Number (Z) | Mass Number (A) |
|---|---|---|---|---|---|
| Hydrogen | 1 | 0 | 1 | 1 | 1 |
| Helium | 2 | 2 | 2 | 2 | 4 |
| Lithium | 3 | 4 | 3 | 3 | 7 |
| Beryllium | 4 | 5 | 4 | 4 | 9 |
| Boron | 5 | 6 | 5 | 5 | 11 |
| Carbon | 6 | 6 | 6 | 6 | 12 |
Of course, since neutral atoms have to have one electron for every proton, an element’s atomic number also tells you how many electrons are in a neutral atom of that element. So, the answer to the question, “What ion has 8 protons, 8 neutrons and 8 electrons?” is O^2- (Oxygen with two negative charges).
Does oxygen have 8 or 10 electrons?
Atoms always strive to have a full outermost electron shell, which for oxygen is 8 electrons. Oxygen is an element with an atomic number of 8, meaning it has 8 protons and 8 electrons. However, when oxygen forms an ion with a #2^-# charge, it gains 2 electrons, making 10 electrons in total. So, while oxygen atoms usually have 8 electrons, they can also have 10 electrons in certain cases.
Understanding the Octet Rule
The octet rule is a fundamental concept in chemistry that explains why atoms tend to have 8 valence electrons. It states that atoms strive to achieve a full outermost electron shell by forming chemical bonds with other atoms. This means that atoms can gain or lose electrons to become more stable and less reactive.
How Many Valence Electrons Does Oxygen Have?
The total number of valence electrons present in an oxygen atom is six. This is because oxygen has an atomic number of 8, which means it has 8 protons and 8 electrons. Of these 8 electrons, the first two are located in the innermost shell and are not involved in chemical bonding. This leaves 6 electrons in the outermost shell, which are the valence electrons.
Can Oxygen Hold 8 Electrons?
In a neutral oxygen atom, there are 8 electrons. However, when oxygen forms a covalent bond with another atom, it can sometimes gain 2 more electrons. This is because oxygen can form double or triple bonds with other atoms, allowing it to share more than one electron. In total, oxygen will have 4 electrons from the 2 bonds and 4 electrons from its 2 lone pairs which adds up to 8 electrons. Hence oxygen is octet and obeys octet rule.
Why Do Atoms Always Want 8 Valence Electrons?
Atoms are most stable, least reactive, when their outermost electron shell is full. This is because the electrons in the outermost shell are the ones that are involved in the formation of chemical bonds. When an atom has 8 valence electrons, it means that it can form maximum number of bonds with other atoms and is less likely to be reactive.
To summarize, oxygen atoms usually have 8 electrons, but in certain cases, such as when they form an ion with a #2^-# charge, they can have 10 electrons. This is because oxygen follows the octet rule, which states that atoms strive to achieve a full outermost electron shell by forming chemical bonds with other atoms. This helps them to become more stable and less reactive.
What element has 8 protons 8 neutrons and 10 electrons?
Atoms of different elements vary in the number of protons, neutrons, and electrons they contain. You can identify an element by its atomic number, which is the number of protons in its nucleus. Knowing the number of protons, neutrons, and electrons in an atom can help you determine its identity.
So, what element has 8 protons, 8 neutrons, and 10 electrons? This is the element carbon, which has an atomic number of 6 and a mass number of 12. Carbon is a very important element, as it is the basis of life on Earth.
Atomic Number (Z)
The number of protons in an atom is called its atomic number ((Z)). This number is very important because it is unique for atoms of a given element. All atoms of an element have the same number of protons, and every element has a different number of protons in its atoms. For example, all helium atoms have two protons, and no other elements have atoms with two protons.
Mass Number (A)
The total number of protons and neutrons in an atom is called its mass number ((A)). This number can be used to calculate the number of neutrons in an atom, since protons and neutrons each count for one AMU (atomic mass unit).
For example, the atomic mass of oxygen is 16. This means we have a total of 16 protons and neutrons. 16 total protons and neutrons minus 8 protons leaves us with 8 neutrons.
Table (PageIndex{1}): Atoms of the First Six Elements
Hydrogen 1 0 1 1 1
Helium 2 2 2 2 4
Lithium 3 4 3 3 7
Beryllium 4 5 4 4 9
Boron 5 6 5 5 11
Carbon 6 6 6 6 12
Of course, since neutral atoms have to have one electron for every proton, an element’s atomic number also tells you how many electrons are in a neutral atom of that element.
Atomic Mass of an Element
The atomic mass of an element is an average over all of the isotopes of each element. This calculation can get surprisingly tricky! For example, the element carbon has two naturally occurring isotopes: 12C and 13C. 12C has 6 protons, 6 neutrons, and 6 electrons, while 13C has 6 protons, 7 neutrons, and 6 electrons.
The average of these two isotopes gives us an atomic mass of 12.0107. This means that an average atom of carbon has 6 protons, 6.0107 neutrons, and 6 electrons.
In conclusion, the element with 8 protons, 8 neutrons, and 10 electrons is carbon, with an atomic number of 6 and a mass number of 12. The atomic mass of carbon is 12.0107, which is the average of the two naturally occurring isotopes of carbon.



Leave a Comment