Have you ever wondered what element is made up of 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6? Do you know what element is made up of 1s2 2s2 2p6 3s2 3p6 4s2 3d3? Or how about 1s 2s 2p 3s 3p? If you’ve ever asked yourself any of these questions, then you’re in the right place!
In this blog post, we’ll explore the elements that make up these configurations and what they mean. We’ll also discuss the atomic weight, number, and group of each element. By the end of this post, you’ll have a basic understanding of the elements that make up these configurations and why they’re important.
Let’s start with the element of 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6. This configuration is made up of two elements, Iridium and Tantalum. Iridium has an atomic weight of 192.2, an atomic number of 77, and is in the transition elements group. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d7.
Tantalum is the other element in this configuration and its atomic weight is 180.9, its atomic number is 73, and it is also in the transition elements group. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d3.
Now let’s move on to the element of 1s2 2s2 2p6 3s2 3p6 4s2 3d3. This configuration is made up of two elements, Iridium and Nickel. Iridium has an atomic weight of 192.2, an atomic number of 77, and is in the transition elements group. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d7.
Nickel has an atomic weight of 58.7, an atomic number of 28, and is in the transition elements group. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d8.
The element of 1s 2s 2p 3s 3p is made up of two elements, Oxygen and Neon. Oxygen has an atomic weight of 15.99, an atomic number of 8, and is in the non-metallic elements group. Its electron configuration is 1s2 2s2 2p4.
Neon has an atomic weight of 20.18, an atomic number of 10, and is in the noble gases group. Its electron configuration is 1s2 2s2 2p6.
The element of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 is made up of two elements, Iridium and Palladium. Iridium has an atomic weight of 192.2, an atomic number of 77, and is in the transition elements group. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d7.
Palladium has an atomic weight of 106.4, an atomic number of 46, and is in the transition elements group. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2.
Finally, the element of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3 is made up of three elements, Iridium, Palladium, and Nickel. Iridium has an atomic weight of 192.2, an atomic number of 77, and is in the transition elements group. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d7.
Palladium has an atomic weight of 106.4, an atomic number of 46, and is in the transition elements group. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2.
Nickel has an atomic weight of 58.7, an atomic number of 28, and is in the transition elements group. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d8.
So, there you have it—the elements that make up 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6, 1s2 2s
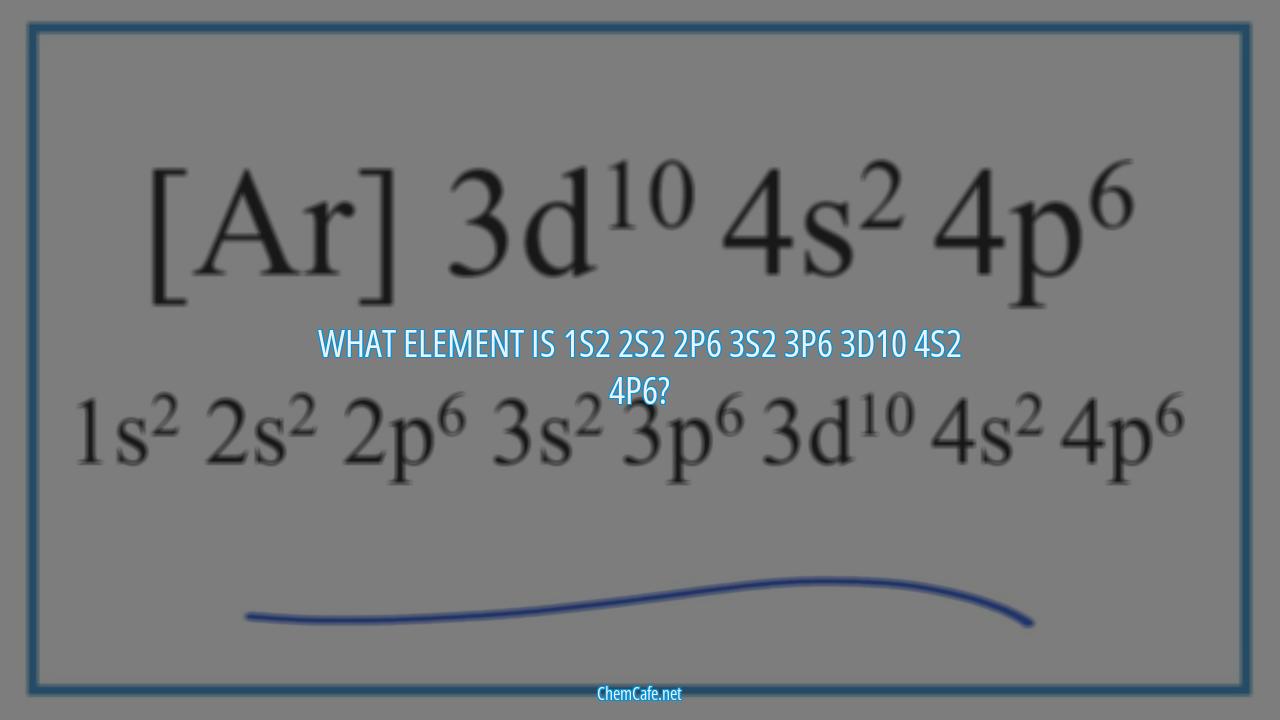
What element is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6?
The element with electron configuration 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 is iridium (Ir). Iridium is a transition metal located in Period 6 and Group 9 of the periodic table. It has an atomic number of 77 and an atomic weight of 192.2.
Exploring the Electron Configuration of Iridium
The electron configuration of iridium is a way of representing the number of electrons in each of its orbitals. It is written in the form 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6. This means that iridium has two electrons in the 1s orbital, two electrons in the 2s orbital, six electrons in the 2p orbital, two electrons in the 3s orbital, six electrons in the 3p orbital, ten electrons in the 3d orbital, two electrons in the 4s orbital, and six electrons in the 4p orbital.
What Does the Electron Configuration Tell Us?
The electron configuration of iridium tells us a lot about its chemical properties. For example, the 2s2 configuration indicates that iridium has two valence electrons in its outer shell. This means that it is likely to form bonds with other elements, as it has the potential to gain or lose electrons. In addition, the 3d10 configuration tells us that iridium has ten electrons in its outermost d orbitals. This gives it a high reactivity and makes it a good catalyst.
Other Elements with Similar Electron Configurations
Iridium is not the only element with an electron configuration of 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6. Other elements with the same configuration include hafnium (Hf), ruthenium (Ru), osmium (Os), and tantalum (Ta). All of these elements are transition metals located in Period 6 and Group 9 of the periodic table. They all have an atomic number of 77 and an atomic weight of 192.2.
In conclusion, the element with electron configuration 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 is iridium. This configuration tells us that iridium has two valence electrons in its outermost shell and ten electrons in its outermost d orbitals. It is a transition metal located in Period 6 and Group 9 of the periodic table. Other elements with the same electron configuration include hafnium, ruthenium, osmium, and tantalum.
What element is 1s2 2s2 2p6 3s2 3p6 4s2 3d3?
In chemistry, the electron configuration of an element is the arrangement of its electrons in different shells and orbitals. It is the basis for understanding many physical and chemical properties of an element. To represent the electron configuration, the elements are organized into periods and groups. Each period is represented by a row in the periodic table and each group is represented by a column.
When looking at the electron configuration of an element, it is important to understand its position in the periodic table. For example, element A is located in Period 2, the 6th position in 2p-block. This means that A has two valence electrons in 2s (2s2) and five valence electrons in 2p (2p5). Answer: 2s22p5. It has 2 + 5 = 7 valence electrons. Element B is located in Period 3, the 2nd position in 3s-block. This means that B has two valence electrons in 3s (3s2). Answer: 3s2.
Element C is located in Period 5, the 1st position in 5s-block). This means that there is only one valence electron in 5s (5s1). Answer: 5s1.
Now, if we look at the electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d3, then we can see that this element is located in Period 4, the 3rd position in 3d-block. This means that it has two valence electrons in 4s (4s2) and three valence electrons in 3d (3d3). Answer: 4s23d3. It has 2 + 3 = 5 valence electrons.
So, the element with the electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d3 is Tantalum (Ta). Its atomic weight is 180.9, atomic number is 73 and it belongs to the transition element group. Its full electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d3.
Another element with electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d7 is Iridium (Ir). Its atomic weight is 192.2, atomic number is 77 and it also belongs to the transition element group. Its full electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d7.
To summarize, the electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d3 is associated with the element Tantalum and the electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d7 is associated with the element Iridium. Both elements belong to the transition elements group and they both have five valence electrons.
Understanding the electron configuration of an element is a powerful tool for understanding its chemical and physical properties. Knowing the atomic number, atomic weight and the group of an element is also important for understanding its properties. With this knowledge, we can better understand the behavior and reactivity of elements.
What is the element of 1s 2s 2p 3s 3p?
Element configuration is an essential part of understanding the periodic table and the elements that make up our universe. The element of 1s 2s 2p 3s 3p is a reference to the number of electrons found in each shell of an atom, and it can help us to understand the properties of different elements.
Atoms are composed of protons, neutrons, and electrons. Protons and neutrons are found in the nucleus of an atom, while electrons are found in the electron shells surrounding the nucleus. Electron shells are referred to by their “n” values, with the lowest energy level being the “1” shell, and the highest energy level being the “4” shell.
To understand the element of 1s 2s 2p 3s 3p, it’s important to understand how electrons fill the shells. Electrons will fill the shells in order of increasing energy, starting with the 1s shell and ending with the 4s shell. Each shell can only hold a certain number of electrons, and the maximum number of electrons that can fill each shell is determined by the “n” value of the shell.
The 1s shell can only hold two electrons, the 2s shell can hold two electrons, and the 2p shell can hold six electrons. The 3s shell can hold two electrons and the 3p shell can hold six electrons. This means that the element of 1s 2s 2p 3s 3p has a total of seven valence electrons.
The element of 1s 2s 2p 3s 3p is a reference to the elements in the first three rows of the periodic table. The first row contains two elements, hydrogen and helium, since two electrons are needed to fill the 1s subshell. This is followed by the second row p-block, containing 6 elements (B through Ne) since six electrons are required to fill the 2p subshell. The third row is similar to the second row elements. Two electrons are needed (Na and Mg) to fill the 3s subshell and six electrons are required (Al through Ar) to complete the 3p subshell.
These elements make up the majority of the elements in the periodic table, and they are all characterized by having seven valence electrons. This means that they are all capable of forming chemical bonds with other elements, and they are all essential components of our universe.
The element of 1s 2s 2p 3s 3p is an important concept to understand when studying the periodic table and the elements that make up our universe. It is a reference to the number of electrons found in each shell of an atom and it can help us to understand the properties of different elements. Understanding this concept is essential for anyone interested in learning more about chemistry and the elements that make up our universe.
What element is 1s2 2s2 2p6 3s2 3p6 4s2 3d10?
The electron configuration of an atom is a representation of the number of electrons in each of its energy levels. The symbol 1s2 2s2 2p6 3s2 3p6 4s2 3d10 is the electron configuration of two elements, A and B. To determine which element A and B is, we need to look at the number of valence electrons each has.
Element A
Element A is located in Period 2, the 2nd position in 2s-block. This means that A has two valence electrons in 2s (2s2) and five valence electrons in 2p (2p5). Therefore, the electron configuration for Element A is 2s22p5. It has 2 + 5 = 7 valence electrons.
Element B
Element B is located in Period 3, the 2nd position in 3s-block. This means that B has two valence electrons in 3s (3s2). Therefore, the electron configuration for Element B is 3s2. It has 2 valence electrons.
Element C
Element C is located in Period 5, the 1st position in 5s-block. This means that there is only one valence electron in 5s (5s1). Therefore, the electron configuration for Element C is 5s1. It has 1 valence electron.
What elements are 1s2 2s2 2p6 3s2 3p6 4s2 3d10?
The electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 corresponds to two elements, Tantalum (Ta) and Iridium (Ir).
Tantalum (Ta)
Tantalum is a transition metal located in Group 5 of the periodic table. It has an atomic weight of 180.9 and an atomic number of 73. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d3.
Iridium (Ir)
Iridium is a transition metal located in Group 7 of the periodic table. It has an atomic weight of 192.2 and an atomic number of 77. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d7.
In conclusion, the electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 corresponds to the two elements Tantalum (Ta) and Iridium (Ir).
What element is 1s2 2s2 2p6 3s2 3p6?
We all know that the periodic table is an essential part of our daily lives. It is a tool used to identify and classify elements based on their atomic number and electronic configuration. Knowing this information is important as it helps us to determine the properties of an element and its role in nature.
One of the most common questions asked is “What element is 1s2 2s2 2p6 3s2 3p6?” The answer is titanium (Ti). Titanium is a transition metal located in the fourth period of the periodic table. It is a silver-gray metal that is strong and resistant to corrosion. It is also used in a variety of applications due to its unique properties.
Understanding Electronic Configuration
Before we can answer the question “What element is 1s2 2s2 2p6 3s2 3p6?,” it is important to understand what electronic configuration is and how it works. Electronic configuration is the arrangement of electrons in an atom. It is usually written in the form of a series of numbers and letters, such as 1s2 2s2 2p6 3s2 3p6.
The first number, 1, represents the first energy level of the atom. The letter s stands for the s orbital, which is the first orbital in the energy level. The second number, 2, represents the number of electrons in the s orbital. The letter p stands for the p orbital, which is the second orbital in the energy level. The third number, 6, represents the number of electrons in the p orbital.
Element with electronic configuration 1s2 2s2 2p6
The element with an electronic configuration of 1s2 2s2 2p6 is nitrogen (N). Nitrogen is located in group 15 of the periodic table and has an atomic number of 7. It is a nonmetal element that is colorless and odorless. It is the most abundant element in the atmosphere, making up about 78% of the air we breathe.
Element with electronic configuration 1s2 2s2 2p6 3s2 3p5
The element with an electronic configuration of 1s2 2s2 2p6 3s2 3p5 is chlorine (Cl). It belongs to group 17 in the periodic table, also known as the halogen group. Chlorine is a greenish-yellow gas that is highly reactive and can be poisonous in large amounts. It is found in nature as a salt in seawater and is used in a variety of industrial and consumer products.
Element with electronic configuration 1s2 2s2 2p6 3s2 3p6 4s2
The element with an electronic configuration of 1s2 2s2 2p6 3s2 3p6 4s2 is titanium (Ti). It is a transition metal located in the fourth period of the periodic table. Titanium is a silver-gray metal that is strong and resistant to corrosion. It is used in a variety of applications due to its unique properties, such as medical implants and industrial machinery.
Group 4A on the periodic table
Group 4A (or IVA) of the periodic table includes the nonmetal carbon (C), the metalloids silicon (Si) and germanium (Ge), the metals tin (Sn) and lead (Pb), and the yet-unnamed artificially-produced element ununquadium (Uuq). These elements are all located in period 4 of the periodic table and have four valence electrons.
In conclusion, the element with an electronic configuration of 1s2 2s2 2p6 3s2 3p6 is titanium (Ti). Titanium belongs to group 4A of the periodic table and has seven valence electrons. It is a strong and corrosion-resistant metal that is used in a variety of applications due to its unique properties. By understanding the properties of titanium and its role in nature, we can better appreciate the importance of the periodic table and its elements.
What is this element 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3?
Atoms can be made up of different elements. Understanding the structure of an element is important for learning about its properties. One way to describe an element’s structure is to look at its electron configuration.
An electron configuration describes the arrangement of electrons in the shells of an atom. It is written using the symbol of the element, and the number of electrons in each shell. For example, the electron configuration of fluorine is 1s2 2s2 2p5. This means that fluorine has two valence electrons in 2s (2s2) and five valence electrons in 2p (2p5).
Element A:
Element A is located in Period 3, the 2nd position in 3s-block. This means that A has two valence electrons in 3s (3s2). Answer: 3s2.
Element B:
Element B is located in Period 5, the 1st position in 5s-block). This means that there is only one valence electron in 5s (5s1). Answer: 5s1.
Examples of Elements and their Electron Configurations:
Rh (Rhodium)
Name of Element : Rhodium
Atomic Weight : 102.9
Atomic Number : 46
Group : Transition Elements
Electron Configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d7
Pd (Palladium)
Name of Element : Palladium
Atomic Weight : 106.4
Atomic Number : 47
Group : Transition Elements
Electron Configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d8
Ir (Iridium)
Name of Element : Iridium
Atomic Weight : 192.2
Atomic Number : 77
Group : Transition Elements
Electron Configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d7
Understanding electron configurations is an important part of understanding the structure of atoms and how they interact with each other. Knowing the electron configuration of an element can help you understand its chemical properties and reactivity. It can also help you predict the behavior of molecules and compounds.
The electron configuration of an element can be determined by looking at its position in the Periodic Table of Elements. The number of electrons in each shell is determined by the number of protons in the nucleus of the atom.
For example, the electron configuration of oxygen is 1s2 2s2 2p4. This means that oxygen has two valence electrons in 2s (2s2) and four valence electrons in 2p (2p4).
By understanding the electron configuration of an element, you can gain a better understanding of its properties and behavior. This knowledge can help you predict how an element will react with other elements, and can also help you understand why certain elements are more or less reactive than others.
Learning about electron configurations is an important part of any chemistry course. With a better understanding of electron configurations, you can better understand the structure of atoms and their behavior.




Leave a Comment