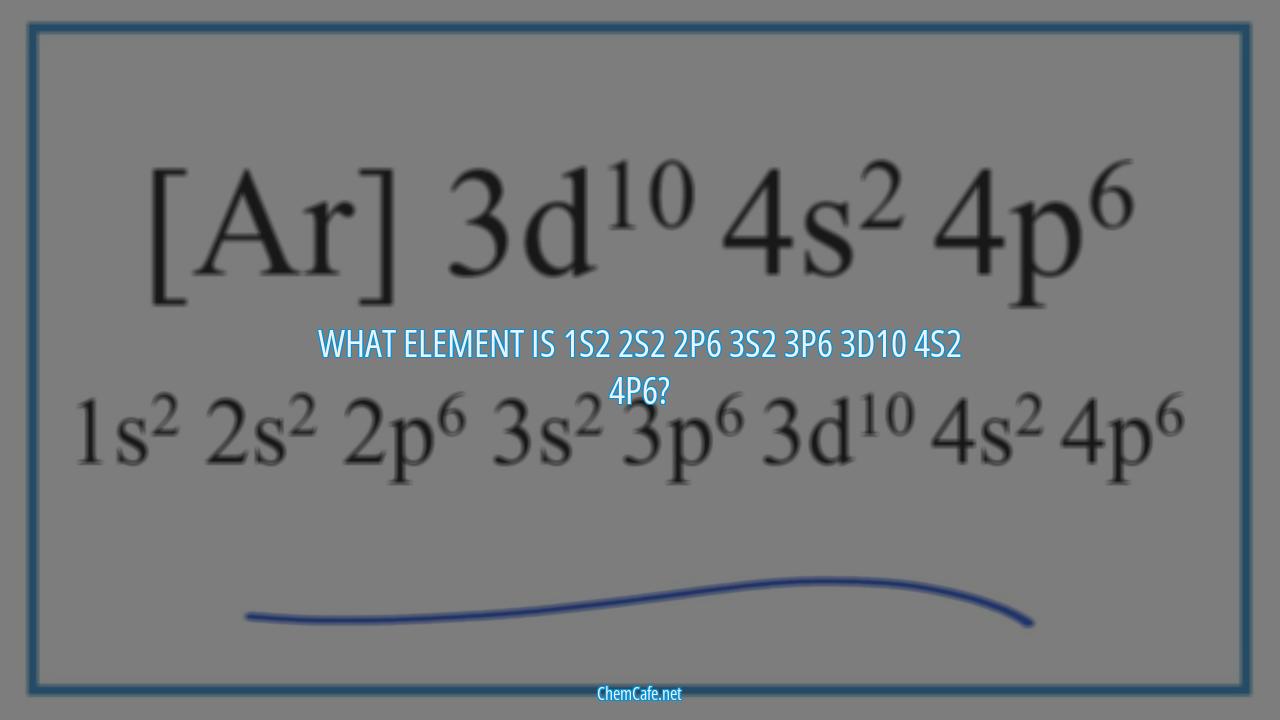
Welcome to the wonderful world of elements! Do you ever find yourself wondering what element is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6? Or maybe you’re curious about 1s2 2s2 2p6 3s2 3p6 4s2 3d3? Or even 1s2 2s2 2p2 3s2 3p6 4s2 3d6?
Elements are the building blocks of the world, and understanding them can be incredibly rewarding. In this blog post, we’ll explain what elements are, what the different configurations mean and how to identify them. We’ll also provide information on the elements associated with 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6, 1s2 2s2 2p6 3s2 3p6 4s2 3d3, 1s2 2s2 2p2 3s2 3p6 4s2 3d6, 1s2 2s2 2p6 3s2 3p6 and 1s22s22p63s23p64s13d5.
Elements are the most basic form of matter, and they are composed of atoms. An atom is the smallest unit of matter that can exist, and it is composed of protons, neutrons and electrons. Protons and neutrons make up the nucleus, and electrons orbit the nucleus in shells. The electrons are arranged in a specific pattern, which is known as the electron configuration. The electron configuration provides information about the atom’s structure, and it can be used to identify the element.
Each element has a unique electron configuration, and the arrangement of electrons can be described using the shell model. In this model, electrons are arranged into shells, and each shell is divided into sub-shells. Each shell can hold a certain number of electrons, and each sub-shell can hold a certain number of electrons. The shells are identified by a number, and the sub-shells are identified by a letter. For example, the 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 configuration indicates that the element has two electrons in the first shell, two electrons in the second shell, six electrons in the third shell, two electrons in the fourth shell and six electrons in the fifth shell.
Now that you know the basics of the shell model, let’s examine the elements associated with 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6, 1s2 2s2 2p6 3s2 3p6 4s2 3d3, 1s2 2s2 2p2 3s2 3p6 4s2 3d6, 1s2 2s2 2p6 3s2 3p6 and 1s22s22p63s23p64s13d5.
The element associated with 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 is Yttrium. It has an atomic weight of 88.9 and an atomic number of 39. It is a transition element and its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d1.
The element associated with 1s2 2s2 2p6 3s2 3p6 4s2 3d3 is Zirconium. It has an atomic weight of 91.2 and an atomic number of 40. It is a transition element and its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d2.
The element associated with 1s2 2s2 2p2 3s2 3p6 4s2 3d6 is Manganese. It has an atomic weight of 54.9 and an atomic number of 25. It is a transition element and its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d5.
The element associated with 1s2 2s2 2p6 3s2 3p6 4s2 3d10 is Niobium. It has an atomic weight of 92.9 and an atomic number of 41. It is a transition element and its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d3.
The element associated with 1s2 2s2 2p6 3s2 3p6 is Molybdenum. It has an atomic weight of 95.9 and an atomic number of 42. It is a transition element and its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d4.
The element associated with 1s22s22p63s23p64s13d5 is Palladium. It has an atomic weight of 106.4 and an atomic number of 47. It is a transition element and its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4
What element is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6?
The element with the electron configuration 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 is Yttrium. Yttrium is a transition element with an atomic weight of 88.9 and an atomic number of 39. Yttrium is part of the transition metals group and is one of the 15 elements in the fourth period of the periodic table.
Yttrium
Yttrium is a soft, silver-metallic transition metal that is known for its use in lasers, TV displays, and other electronic components. Yttrium is also used in medical imaging, as it has high neutron absorption cross-sections and can be used to detect cancer cells. Yttrium is also used in the production of superconductors, as it has a high critical temperature.
Atomic Weight and Number
Yttrium has an atomic weight of 88.9 and an atomic number of 39. This means that yttrium has 39 protons and 39 electrons. Yttrium has a valence shell configuration of 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6, which is the same as its electron configuration.
Group
Yttrium belongs to the transition elements group, which is also known as the transition metals. This group of elements consists of metals that are located in the middle of the periodic table and have a variety of chemical and physical properties. These elements are characterized by their ability to form compounds with multiple oxidation states.
Other Transition Elements
Other transition elements include Zirconium (atomic weight 91.2, atomic number 40), Niobium (atomic weight 92.9, atomic number 41), Molybdenum (atomic weight 95.9, atomic number 42), Manganese (atomic weight 54.9, atomic number 25), Palladium (atomic weight 106.4, atomic number 47), Silver (atomic weight 107.9, atomic number 47), Cadmium (atomic weight 112.4, atomic number 48), Indium (atomic weight 114.8, atomic number 49), Tin (atomic weight 118.7, atomic number 50), Antimony (atomic weight 121.8, atomic number 51), Tellurium (atomic weight 127.6, atomic number 52), Iodine (atomic weight 126.9, atomic number 53), Xenon (atomic weight 131.3, atomic number 54), Cesium (atomic weight 132.9, atomic number 55), Radon (atomic weight 222.0, atomic number 86), Francium (atomic weight 223.0, atomic number 87), Radium (atomic weight 226.0, atomic number 88), and Actinium (atomic weight 227.0, atomic number 89).
Each of these elements has a unique electron configuration and belongs to the transition elements group. The transition elements group is characterized by its ability to form compounds with multiple oxidation states and its variety of chemical and physical properties.
Uses of Yttrium
Yttrium is used in a variety of applications, including lasers, TV displays, medical imaging, and the production of superconductors. Yttrium is also used to make phosphors and catalysts, and it is often used as an alloying agent in various metals and alloys. Yttrium is also used in the production of glass and ceramics, and it is used in the manufacture of nuclear reactors and nuclear fuels.
The element with the electron configuration 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 is yttrium. Yttrium is a soft, silver-metallic transition metal that is part of the transition elements group and has an atomic weight of 88.9 and an atomic number of 39. Yttrium is used in a variety of applications, including lasers, TV displays, medical imaging, and the production of superconductors. Yttrium is also used to make phosphors and catalysts, and it is often used as an alloying agent in various metals and alloys.
What element is 1s2 2s2 2p6 3s2 3p6 4s2 3d3?
The electron configuration of an element is a representation of the arrangement of electrons around the nucleus of an atom. It is an important concept in chemistry, and can be used to identify an element and predict its properties, including its reactivity and its bond strengths. The most common form of electron configuration is the one-electron configuration, where all of the electrons of an element are arranged in shells around the nucleus. The 1s2 2s2 2p6 3s2 3p6 4s2 3d3 electron configuration is the configuration of the element palladium.
Palladium (symbol: Pd) is a silvery-white metal that is part of the transition metals group. It is a rare element that has many uses in industry, including in catalysts and electronics. It is also used in jewelry and coins, and is sometimes used as a substitute for platinum. Palladium is highly reactive and has a melting point of 1554.9 °C (1830.6 °F).
The 1s2 2s2 2p6 3s2 3p6 4s2 3d3 electron configuration can be broken down into the following parts:
1s2
This is the first shell of electrons around the nucleus. It is filled with two electrons, which are in the 1s orbital. This orbital is located at the closest distance to the nucleus, so the electrons in this shell are the most tightly bound to the nucleus and experience the most attraction.
2s2
The 2s shell is the second shell of electrons around the nucleus. It is filled with two electrons, which are in the 2s orbital. This orbital is located at a slightly larger distance from the nucleus, so the electrons in this shell experience less attraction from the nucleus than those in the 1s shell.
2p6
The 2p shell is the third shell of electrons around the nucleus. It is filled with six electrons, which are in the three 2p orbitals. These orbitals are located at a greater distance from the nucleus, so the electrons in this shell experience less attraction from the nucleus than those in the 1s and 2s shells.
3s2
The 3s shell is the fourth shell of electrons around the nucleus. It is filled with two electrons, which are in the 3s orbital. This orbital is located at a greater distance from the nucleus, so the electrons in this shell experience less attraction from the nucleus than those in the 1s, 2s, and 2p shells.
3p6
The 3p shell is the fifth shell of electrons around the nucleus. It is filled with six electrons, which are in the three 3p orbitals. These orbitals are located at a greater distance from the nucleus, so the electrons in this shell experience less attraction from the nucleus than those in the 1s, 2s, 2p, and 3s shells.
4s2
The 4s shell is the sixth shell of electrons around the nucleus. It is filled with two electrons, which are in the 4s orbital. This orbital is located at a greater distance from the nucleus, so the electrons in this shell experience less attraction from the nucleus than those in the 1s, 2s, 2p, 3s, and 3p shells.
3d3
The 3d shell is the seventh shell of electrons around the nucleus. It is filled with three electrons, which are in the three 3d orbitals. These orbitals are located at a greater distance from the nucleus, so the electrons in this shell experience less attraction from the nucleus than those in the 1s, 2s, 2p, 3s, 3p, and 4s shells.
The 1s2 2s2 2p6 3s2 3p6 4s2 3d3 electron configuration is the electron configuration of the element palladium. Palladium is a rare element that has many uses in industry, including in catalysts and electronics. It is also used in jewelry and coins, and is sometimes used as a substitute for platinum. Palladium is highly reactive and has a melting point of 1554.9 °C (1830.6 °F).
When looking at the electron configuration of an element, it is important to remember that each shell can only hold a certain number of electrons. For example, the 1s shell can only hold two electrons, the 2s shell can only hold two electrons, and so on. Each electron must occupy its own orbital, so the 1s shell cannot hold more than two electrons, the 2s shell cannot hold more than two electrons, and so on. This is why it is important to understand electron configurations, as they can help to identify elements and predict their properties.
What is the element of 1s 2s 2p 3s 3p?
Atoms are composed of electrons, protons and neutrons. Electrons are arranged around the nucleus in shells or orbitals. The electron configuration of an atom is a representation of the arrangement of its electrons in various orbitals. The element of 1s 2s 2p 3s 3p is the electron configuration of an atom with five electrons.
The electron configuration of an atom is determined by the energy levels and sublevels of the electron orbitals. The energy levels are determined by the principal quantum number (n) and the sublevels are determined by the azimuthal quantum number (l). In the case of 1s 2s 2p 3s 3p, the principal quantum number is 1 for the 1s orbital, 2 for the 2s and 2p orbitals, and 3 for the 3s and 3p orbitals. The azimuthal quantum number is 0 for the s orbitals and 1 for the p orbitals.
Order of Filling Subshells
The order of filling subshells determines the electron configuration of an atom. The 1s orbital is the first to fill, followed by the 2s and then the 2p orbitals. The 3s and 3p orbitals are next, followed by the 4s and 3d orbitals. The order of filling is then 4p, 5s, 4d, 5p, 6s and so on.
Maximum Number of Electrons in a Subshell
The maximum number of electrons that can be accommodated by a subshell is given by the formula 2*(2l + 1). Therefore, the s, p, d, and f subshells can accommodate a maximum of 2, 6, 10, and 14 electrons, respectively. All the possible subshells for values of n up to 4 are tabulated below.
Principle Quantum Number Value | Value of Azimuthal Quantum Number | Resulting Subshell in the Electron Configuration
n=1 l=0 | 1s
n=2 l=0 | 2s
l=1 | 2p
n=3 l=0 | 3s
l=1 | 3p
l=2 | 3d
n=4 l=0 | 4s
l=1 | 4p
l=2 | 4d
l=3 | 4f
It can be understood that the 1p, 2d, and 3f orbitals do not exist because the value of the azimuthal quantum number is always less than that of the principal quantum number.
Notation
The electron configuration of an atom is written with the help of subshell labels. These labels contain the shell number (given by the principal quantum number), the subshell name (given by the azimuthal quantum number) and the total number of electrons in the subshell in superscript. For example, if two electrons are filled in the ‘s’ subshell of the first shell, the resulting notation is ‘1s2’.
Example
The 4p subshell is filled next by six electrons (Ga through Kr). As you can see, the periodic table shown in Figure (PageIndex{3}) provides a simple way to remember the order of filling the subshells in determining the electron configuration. The order of filling subshells is the same: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, etc.
Example (PageIndex{2}): Aluminum
Using Figure (PageIndex{3}) as your guide, write the electron configuration of neutral aluminum atom. The atomic number of Al is 13.
Solution: Aluminum has 13 electrons. Start at Period 1 of the periodic table, Figure (PageIndex{3}). Place two electrons in the 1s subshell (1s2). Proceed to Period 2 (left to right direction). Place the next two electrons in the 2s subshell (2s2) and the next six electrons in the 2p subshell (2p6). Proceed to Period 3 (left to right direction). Place the next two electrons in the 3s subshell (3s2) and the last one electron in the 3p subshell (3p1). The electron configuration of Aluminum is 1s22s22p63s23p1
Using Figure (PageIndex{3}) as your guide, write the electron configuration of the atom that has 20 electrons
Answer: Start at Period 1 of Figure (PageIndex{3}). We can also use the block organization of the periodic table, as shown below, to remind us of the order in which sublevels are filled. To do this, we move through the elements in the order of increasing atomic number, listing new sublevels as we come to them. The type of sublevel (s, p, d, or f ) is determined from the block in which the atomic number is found. The number for the principal energy level (for example, the 3 in 3p) is determined from the row in which the element is found and the knowledge that the s sublevels start on the first principal energy level, the p sublevels start on the second principal energy level, the d sublevels start on the third principal energy level, and the f sublevels start on the fourth principal energy level.
We know that the first two electrons added to an atom go to the 1s sublevel. Atomic numbers 3 and 4 are in the second row of the s block (look for them in the bottom half of in image below), signifying that the 3rd and 4th electrons are in the 2s sublevel. The next six electrons go to the 2p sublevel (2p6), followed by the 3s and 3p orbitals (3s2 and 3p6). The last two electrons go to the 4s subshell (4s2). The electron configuration of the atom with 20 electrons is 1s22s22p63s23p64s2.
Understanding the element of 1s 2s 2p 3s 3p is important for understanding the electron configuration of atoms. Knowing the order of filling the subshells and the maximum number of electrons in a subshell can help you to write the electron configuration of an atom quickly and accurately.
What element is 1s2 2s2 2p6 3s2 3p6 4s2 3d10?
We all know the periodic table of elements, but do you ever wonder what each of the elements are made up of? It’s easy to remember the names of each element, but understanding its composition is a different story. It’s important to know what element is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 because it can help you identify elements quickly and accurately.
The element 1s2 2s2 2p6 3s2 3p6 4s2 3d10 is antimony, which is a non-metal with an atomic number of 51 and an atomic weight of 121.8. Antimony is found in nature in the form of sulfide and oxide minerals and is used in metallurgy, electronics, and other industrial applications.
Antimony is a semimetal, meaning it has some metallic characteristics, but it is not considered a metal. It has a shiny, silver-white appearance and is relatively stable in air. It is also highly resistant to corrosion and is a good conductor of electricity.
Antimony’s electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10. This means that the element has five valence electrons in its outermost shell (the 4s2 3d10), which makes it a non-metal. Antimony’s electron configuration also explains why it is a good conductor of electricity, as the five valence electrons are able to easily move around and transfer energy.
The Properties of Antimony
Antimony is a chemical element that can be found in nature in the form of sulfide and oxide minerals. It is a semimetal, meaning it has some metallic characteristics, but it is not considered a metal. Antimony has a shiny, silver-white appearance and is relatively stable in air. It is also highly resistant to corrosion and is a good conductor of electricity.
Antimony is a brittle, crystalline solid at room temperature, and it has a relatively low melting point. It is also relatively non-toxic and is not flammable. Antimony is used in metallurgy, electronics, and other industrial applications.
Common Uses of Antimony
Antimony is used in many different industries, including metallurgy, electronics, and other industrial applications. It is often used in lead-acid batteries, for example, as a hardening agent and in the production of semiconductors. Antimony is also used in the production of flame retardants, glass, and ceramics.
Antimony is also used in medicine, in the form of antimony trisulfide, which is used to treat certain skin diseases. In addition, antimony compounds are used in the production of pigments, dyes, and enamels.
The element 1s2 2s2 2p6 3s2 3p6 4s2 3d10 is antimony, which is a non-metal with an atomic number of 51 and an atomic weight of 121.8. Antimony is found in nature in the form of sulfide and oxide minerals and is used in metallurgy, electronics, and other industrial applications. It has a shiny, silver-white appearance and is relatively stable in air. It is also highly resistant to corrosion and is a good conductor of electricity. Antimony is used in many different industries, including metallurgy, electronics, and other industrial applications. It is also used in medicine, in the form of antimony trisulfide, which is used to treat certain skin diseases. Knowing the electron configuration of antimony can help you quickly and accurately identify elements.
What element is 1s2 2s2 2p6 3s2 3p6?
The electron configuration 1s2 2s2 2p6 3s2 3p6 corresponds to the element phosphorus (P). This element is a non-metal located in group 15 of the periodic table. It has an atomic number of 15, meaning it has 15 protons in its nucleus. It also has 15 electrons, and it is the 15th element on the periodic table.
Phosphorus is a multivalent non-metal of the nitrogen group. It is a very reactive element, and it is rarely found in its pure form. It is an essential component of DNA and RNA, as well as many other biological molecules. It is also a component of many fertilizers and explosives.
What Group is this Element in 1s2 2s2 2p3?
Element with electron configuration 1s2 2s2 2p3 is Nitrogen (N). It belongs to group 15 in the periodic table, which is known as the pnictogens. Nitrogen is a non-metal, and it is one of the most abundant elements in the universe. It is found in the atmosphere as well as in many molecules, such as proteins and enzymes.
What is True about the Element with an Electronic Configuration of 1s2 2s2 2p6 3s2 3p5?
The element with an electronic configuration of 1s2 2s2 2p6 3s2 3p5 belongs to group 17 in the periodic table, or the halogen group. Its name is chlorine.
Chlorine is a very reactive element, and it is found in many compounds. It is used in the production of many industrial products, such as polyvinyl chloride (PVC) and other plastics. It is also used in the production of bleaching agents, disinfectants, and other household products.
Which Element is this 1s2 2s2 2p6 3s2 3p6 4s2?
The electronic configuration of 1s2 2s2 2p6 3s2 3p6 4s2 corresponds to the element titanium (Ti). Titanium is a transition metal located in group 4 of the periodic table. It has an atomic mass of 22, and it has 22 electrons. It is a very strong and lightweight metal, and it is used in the production of aircraft and other aerospace components.
What are Group 1 and 2 Called in the Periodic Table?
Group 1 elements are known as the alkali metals, and Group 2 are the alkaline earth metals. Alkali metals are all soft, shiny and metallic when cut. They react easily with water, have low melting points and densities, and are good conductors of electricity. Alkaline earth metals, on the other hand, are hard and brittle and are less reactive than alkali metals.
Group 17 elements are known as the halogens, and Group 18 are the noble gases. Halogens are very reactive and can form compounds with other elements. Noble gases, on the other hand, are very unreactive and do not form compounds with other elements.
What is Group 1’s Name on the Periodic Table?
Group 1 elements include the alkali metals, Li, Na, K, Rb, Cs, Fr. The alkali metals are all soft, shiny and metallic when cut. They react easily with water, have low melting points and densities, and are good conductors of electricity.
What is the Atomic Number of an Atom with an Electron Configuration of 1s2 2s2 2p6 3s2 3p3?
The electronic configuration 1s2,2s2,2p6,3s2,3p3 represents phosphorus with atomic number 15. It is a non-metal located in group 15 of the periodic table. It has an atomic mass of 30.97, and it has 15 protons and 15 electrons.
How Many Electrons Does 1s2 2s2 2p6 3s2 Have?
You have fourteen electrons. Written out the long way, it looks like 1s2 2s2 2p6 3s2 3p2. Do you see how the numbers add up to fourteen? Row one has a shell that can hold two electrons.
How Many Valence Electrons Does 1s2 2s2 2p6 Have?
For the given electron configuration, the valence shell would be the 4th energy level which includes the 4s orbital. Thus, it only has two valence electrons since the 4s orbital has 2 electrons.
Is 1s2 2s2 2p6 Stable?
Yes, the electronic configuration 1s2 2s2 2p6 is stable. This configuration is known as the octet rule, which states that the outermost shell of an atom should contain eight electrons. For this particular configuration, the element is neon.
How Many Valence Electrons are There in Electron Configuration of 1s2 2s2 2p6?
The number of electrons in an atom’s outermost shell are called valence electrons that participate in the formation of a chemical bond. The atom with electronic configuration 1s2 2s2 2p6 3s2 3p2 has 4 valence electrons in its outermost shell.
What are 7 Periods and 18 Groups?
The seven periods of the periodic table are the rows of elements, and the eighteen groups are the columns of elements. Each period contains elements with similar properties, and each group contains elements with similar properties. For example, the elements of Group 1 are known as the alkali metals, Group 2 are the alkaline earth metals, Group 17 are the halogens, and Group 18 are the noble gases.
What Group and Period is 1s2 2s2 2p6?
The outer electronic configuration 2s2 2p6 corresponds to the complete octet. Therefore, the element is placed in the 2nd period of group 18 in the modern periodic table. This element is neon.
What is the Group of 1s2 2s2 2p6?
The group of 1s2 2s2 2p6 is group 18. This is the noble gas group, and it contains the elements helium, neon, argon, krypton, xenon, and radon. These elements are all unreactive, and they are all gases at room temperature.
What does 1s2 2s2 2p6 Mean?
1s2 2s2 2p6 represents two electrons in the s subshell of the first energy level, two electrons in the s subshell of the second energy level, and six electrons in the p subshell of the second energy level. This element has 10 total electrons.
How do You Find the Group and Period?
The period or row numbers 1 through 7 are the energy levels of the elements. The s orbital holds a maximum of 2 electrons. The p orbital can hold 6. The d orbital can hold 10. The f orbital can hold 14 electrons. But, the orbitals overlap. The Madelung rule gives the order:
1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p < 5s < 4d < 5p < 6s < 4f < 5d < 6p < 7s < 5f < 6d < 7p
Oganesson (element 118) is a good example to show the order of the orbitals. Its electron configuration is: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6 7s2 5f14 6d10 7p6
Alternatively, write the symbol for the noble gas before an element (radon, in this case), and just add the extra information: [Rn] 5f14 6d10 7s2 7p6
Keep in mind, electron configurations are most stable when they are filled or half-filled. Also, the real electron configuration of an atom may differ from the prediction because of relativistic effects, shielding, etc.
In conclusion, the electron configuration 1s2 2s2 2p6 3s2 3p6 corresponds to the element phosphorus (P). It is a non-metal located in group 15 of the periodic table. The outer electronic configuration 2s2 2p6 corresponds to the complete octet, and the element is placed in the 2nd period of group 18 in the modern periodic table. Group 1 elements are known as the alkali metals, and Group 2 are the alkaline earth metals. Group 17 elements are known as the halogens, and Group 18 are the noble gases. The number of electrons in an atom's outermost shell are called valance electrons that participate in the formation of a chemical bond. The atom with electronic configuration 1s2 2s2 2p6 3s2 3p2 has 4 valance electrons in its outermost shell. The seven periods of the periodic table are the rows of elements, and the eighteen groups are the columns of elements.
What element has 1s22s22p63s23p64s13d5?
The answer to this question is Silicon, which is the element with atomic number 14. This is because the electron configuration of Silicon is 1s22s22p63s23p64s13d5. This configuration can be determined by following the orbital-filling chart in Figure 5.9 and the electron configurations of the first 18 elements in Table 5.2.
The Aufbau Principle
The Aufbau principle dictates that electrons will occupy the orbitals having lower energies before occupying higher energy orbitals. This principle is named after the German word ‘Aufbeen’ which means ‘build up’. The energy of an orbital is calculated by the sum of the principal and the azimuthal quantum numbers. According to this principle, electrons are filled in the following order: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p.
Box Diagrams of Electron Configuration
If an atom has a partially filled sublevel, it may be important to know how the electrons of that sublevel are distributed among the orbitals. Research has shown that unpaired electrons (a single electron in an orbital) are in a lower energy configuration than are paired electrons (two electrons in an orbital). The energy of the electrons in a sublevel would then be lower with half-filled orbitals than with some filled and some empty. We can show the distribution of electrons by using box diagrams, where each box represents an orbital and the arrows within the boxes represent the electrons in that orbital.
Pauli Exclusion Principle
The Pauli exclusion principle states that a maximum of two electrons, each having opposite spins, can fit in an orbital. This means that the supercripts (2, 2, 6, 2 and 2) in the electron configuration of Silicon add up to 14, which is the atomic number of Silicon.
Filling of Atomic Orbitals
The period or row numbers 1 through 7 are the energy levels of the elements. The s orbital holds a maximum of 2 electrons. The p orbital can hold 6. The d orbital can hold 10. The f orbital can hold 14 electrons. But, the orbitals overlap. The Madelung rule gives the order: 1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p < 5s < 4d < 5p < 6s < 4f < 5d < 6p < 7s < 5f < 6d < 7p. Oganesson (element 118) is a good example to show the order of the orbitals. Its electron configuration is: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6 7s2 5f14 6d10 7p6. Alternatively, write the symbol for the noble gas before an element (radon, in this case), and just add the extra information: [Rn] 5f14 6d10 7s2 7p6. It is important to note that there exist many exceptions to the Aufbau principle such as chromium and copper. These exceptions can sometimes be explained by the stability provided by half-filled or completely filled subshells. Additionally, the real electron configuration of an atom may differ from the prediction because of relativistic effects, shielding, etc. In conclusion, the element with the electron configuration 1s22s22p63s23p64s13d5 is Silicon, which has an atomic number of 14. This can be determined by following the orbital-filling chart in Figure 5.9 and the electron configurations of the first 18 elements in Table 5.2 as well as by understanding the Aufbau principle and the Pauli exclusion principle. Additionally, the Madelung rule gives the order of the orbitals and understanding the exceptions to the Aufbau principle may be important in some cases.
What element is 1s2 2s2 2p2 3s2 3p6 4s2 3d6?
Electron configuration is an important concept in chemistry, as it explains how atoms are arranged in a certain element. The arrangement of electrons is determined by the period or row number of the elements, as well as the different orbitals that can hold electrons. This article will explain the electron configuration of the element with the configuration 1s2 2s2 2p2 3s2 3p6 4s2 3d6.
The element 1s2 2s2 2p2 3s2 3p6 4s2 3d6 is manganese (Mn). Mn is a transition element with an atomic number of 25 and an atomic weight of 54.9. It is in group 7 of the periodic table, which is also known as the transition metals. This element is essential for many biological processes, and it is also used in many industrial applications.
The 1s2 2s2 2p2 3s2 3p6 4s2 3d6 electron configuration can be broken down into its individual components. The 1s2 part of the configuration corresponds to the first energy level, which holds two electrons in the 1s orbital. The 2s2 part of the configuration corresponds to the second energy level, which holds two electrons in the 2s orbital. The 2p2 part of the configuration corresponds to the third energy level, which holds two electrons in the 2p orbital. The 3s2 part of the configuration corresponds to the fourth energy level, which holds two electrons in the 3s orbital. The 3p6 part of the configuration corresponds to the fifth energy level, which holds six electrons in the 3p orbital. The 4s2 part of the configuration corresponds to the sixth energy level, which holds two electrons in the 4s orbital. Finally, the 3d6 part of the configuration corresponds to the seventh energy level, which holds six electrons in the 3d orbital.
Order of the Orbitals
The period or row numbers 1 through 7 are the energy levels of the elements. The s orbital holds a maximum of 2 electrons. The p orbital can hold 6. The d orbital can hold 10. The f orbital can hold 14 electrons. But, the orbitals overlap. The Madelung rule gives the order:
- 1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p < 5s < 4d < 5p < 6s < 4f < 5d < 6p < 7s < 5f < 6d < 7p
Oganesson (element 118) is a good example to show the order of the orbitals. Its electron configuration is: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6 7s2 5f14 6d10 7p6
Alternatively, write the symbol for the noble gas before an element (radon, in this case), and just add the extra information: [Rn] 5f14 6d10 7s2 7p6
Keep in mind, electron configurations are most stable when they are filled or half-filled. Also, the real electron configuration of an atom may differ from the prediction because of relativistic effects, shielding, etc.
In conclusion, the element 1s2 2s2 2p2 3s2 3p6 4s2 3d6 is Manganese (Mn). It is a transition element with an atomic number of 25 and an atomic weight of 54.9. It is in group 7 of the periodic table, which is also known as the transition metals. The electron configuration can be broken down into its individual components, with the order of the orbitals determined by the Madelung rule. It is important to remember that electron configurations are most stable when they are filled or half-filled, and that the real electron configuration of an atom may differ from the prediction due to relativistic effects and shielding.



Leave a Comment