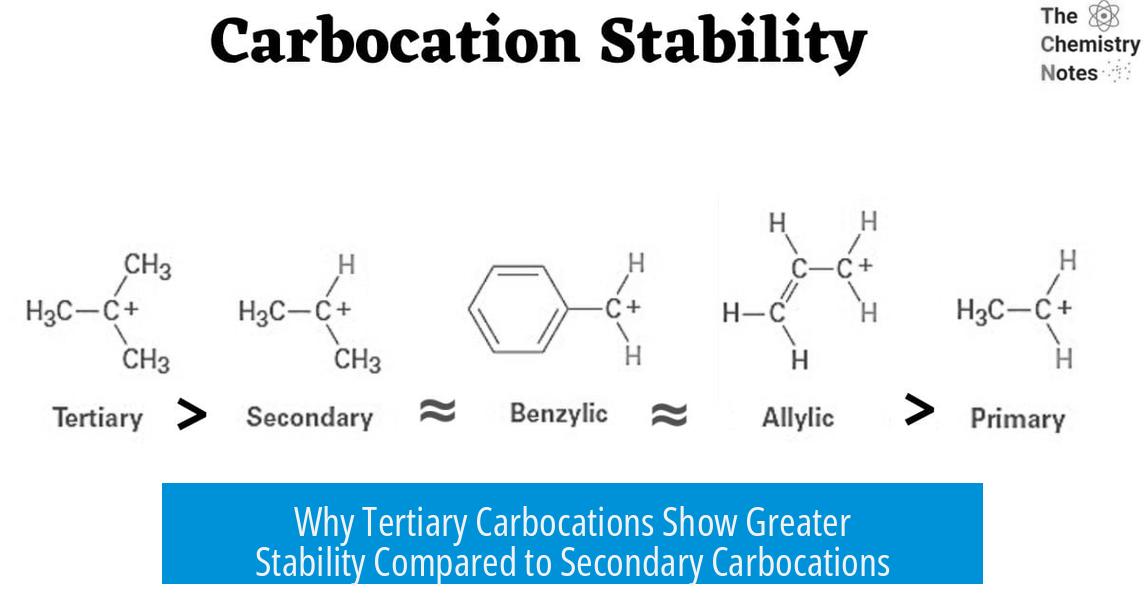
Why Are Tertiary Carbocations So Much More Stable Than Secondary Carbocations?
Tertiary carbocations exhibit significantly higher stability than secondary carbocations due to a combination of electronic and steric factors, including inductive effects, charge diffusion, the sigma (σ) effect, and steric repulsion in the starting material, rather than hyperconjugation alone.
Limitations of Hyperconjugation
Hyperconjugation contributes to carbocation stability by delocalizing charge through adjacent C-H bonds. However, its effect is mainly additive and cannot fully explain the large jump in stability seen in tertiary carbocations compared to secondary ones. If hyperconjugation were the sole reason, the increase in stability would be more gradual rather than pronounced.
Inductive Effect and Charge Diffusion
Alkyl groups attached to the carbocation center stabilize the positive charge via the inductive effect. These groups push electron density through sigma bonds towards the positively charged carbon, reducing its electron deficiency.
This electron donation disperses the positive charge across multiple atoms, a concept similar to “charge diffusion.” The greater the diffusion, the less localized and more stable the carbocation becomes. Tertiary carbocations, with three alkyl groups, distribute the charge more effectively than secondary carbocations, which have only two.
Sigma (σ) Effect
The sigma effect further enhances stability by pushing electron density into the positively charged center directly. This additional electron donation is stronger in tertiary carbocations due to the presence of more alkyl groups.
Steric Repulsion in Starting Materials
Recent studies show steric interactions in the starting alkyl structure influence carbocation stability. Steric repulsion destabilizes the starting material when bulky groups are present, effectively lowering the energy barrier to carbocation formation and resulting in a more stable tertiary carbocation.
Summary Table of Factors Affecting Stability
| Factor | Description | Effect on Stability |
|---|---|---|
| Hyperconjugation | Delocalization through adjacent C-H bonds | Contributes additively but insufficient alone |
| Inductive Effect | Electron donation via sigma bonds from alkyl groups | Disperses positive charge, stabilizes carbocation |
| Sigma Effect | Direct push of electron density into carbocation | Increases electron density and stability |
| Steric Repulsion | Repulsion in starting material lowers formation energy | Facilitates formation and stabilizes tertiary carbocation |
Key Takeaways
- Tertiary carbocations are more stable due to charge dispersion from multiple alkyl groups.
- Inductive effects and the sigma effect push electron density into the carbocation center, enhancing stability.
- Hyperconjugation alone cannot explain the large stability difference.
- Steric repulsion in starting materials also influences stability by lowering energy barriers.
Why does hyperconjugation not fully explain the stability difference between tertiary and secondary carbocations?
Hyperconjugation provides additive stabilization. It cannot account for the large stability jump observed with tertiary carbocations compared to secondary ones.
How does steric repulsion in the starting material affect carbocation stability?
Steric repulsion in the starting material can destabilize certain structures. Tertiary carbocations form in environments where such repulsion favors their stability over secondary ones.
What role does the sigma effect play in stabilizing tertiary carbocations?
The sigma effect pushes electron density into the carbocation center. This electron donation helps reduce the positive charge, making tertiary carbocations more stable.
How does the inductive effect disperse positive charge in tertiary carbocations?
Alkyl groups donate electron density through sigma bonds. This charge diffusion lowers the charge concentration on the carbocation, stabilizing tertiary carbocations versus secondary ones.
Is charge diffusion critical for carbocation stability?
Yes, spreading the positive charge over multiple atoms reduces its energy. This charge distribution stabilizes tertiary carbocations more than secondary carbocations.



Leave a Comment