Why Does BH3•THF Not Reduce Ketones as Well as Carboxylic Acids?

BH3•THF preferentially reduces carboxylic acids over ketones due to its electrophilic nature and selectivity toward more electron-rich functional groups. This difference in reactivity allows selective reduction of carboxylic acids without significantly affecting ketones under controlled conditions.
Reactivity of BH3•THF as a Reducing Agent
Borane (BH3) complexed with tetrahydrofuran (THF) acts as an electrophilic reducing agent. It targets electron-rich sites in molecules. Carboxylic acids contain hydroxyl and carbonyl groups, which create a polarized environment making the acid more reactive toward BH3•THF.
Ketones, however, have less polarity and are less electron-rich compared to carboxylic acids. This lowers their reactivity with BH3•THF, so ketone reduction happens at a slower rate.
Selectivity Between Carboxylic Acids and Ketones
- BH3•THF reacts quickly with carboxylic acids due to favorable interaction with acidic protons and carbonyl oxygen.
- Ketone carbonyls are less reactive to BH3•THF because they lack acidic hydrogens and are electronically less attractive.
- This selectivity allows reduction of carboxylic acids in the presence of ketones if BH3 is used in stoichiometric amounts and not excess.
- Excess BH3•THF can eventually reduce the ketone as well, defeating the selectivity.
Alternative Approach to Preserve Ketones During Reduction

When selective reduction is essential, protecting the ketone can be advantageous. One common method is acetal formation using ethylene glycol and acid catalysis:
- Protect the ketone as an acetal.
- Reduce the carboxylic acid using LiAlH4, which does not affect the acetal.
- Remove the acetal protecting group using acidic hydrolysis to regenerate the ketone.
This method prevents unwanted ketone reduction and provides access to the desired alcohol selectively from the acid.
Key Points
- BH3•THF is an electrophilic reagent, favoring more electron-rich carboxylic acids over ketones.
- Selective reduction occurs due to differing polarities and reactivities of functional groups.
- Using stoichiometric BH3 avoids reducing ketones when carboxylic acids are present.
- Protecting ketones as acetals allows more robust reduction of carboxylic acids with stronger reagents like LiAlH4.
Why Does BH3•THF Not Reduce the Ketone as Well as the Carboxylic Acid?
In a nutshell, BH3•THF preferentially reduces carboxylic acids over ketones due to borane’s electrophilic nature, which makes it react faster with the more nucleophilic (electron-rich) carboxyl group rather than the ketone’s carbonyl. This selectivity means that under controlled conditions, carboxylic acids are reduced smoothly while ketones remain relatively untouched.
Sounds a bit counterintuitive at first, right? After all, we know ketones are generally reactive carbonyl compounds. But the subtle interplay between electronic factors and reagent behavior is what gives borane its selective punch.
The Electrophilic Behavior of Borane (BH3•THF)
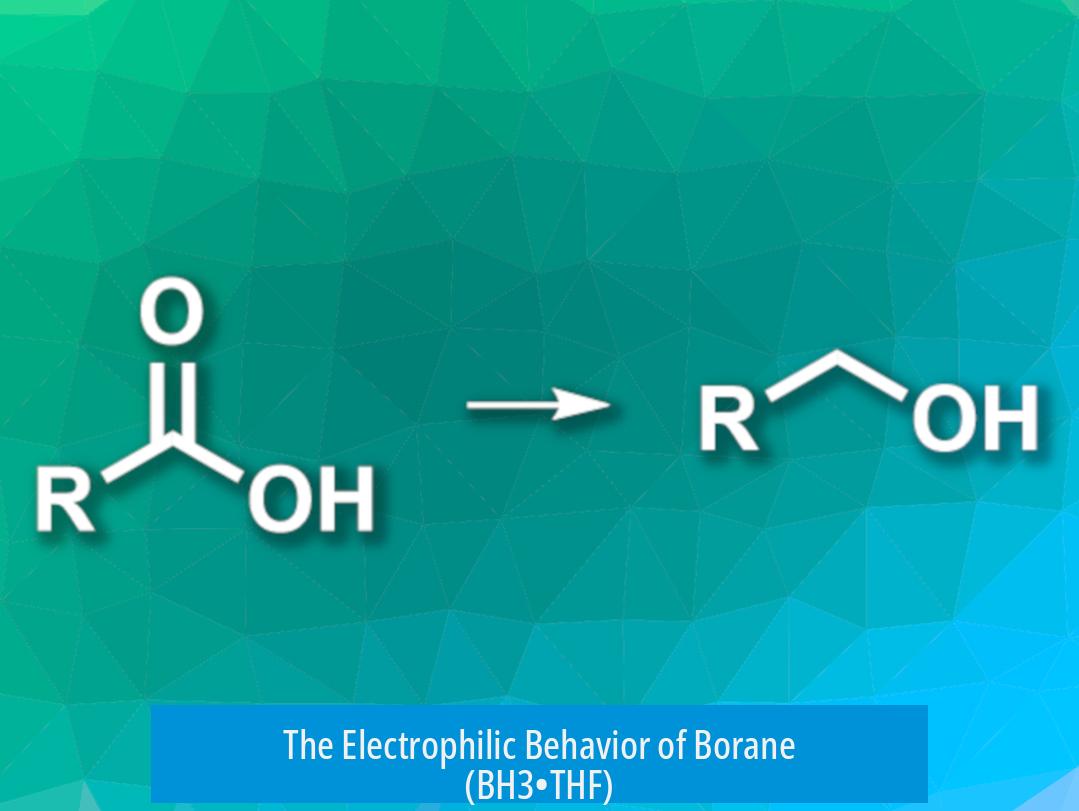
Let’s kick things off with the personality of borane. BH3 (when complexed with THF to stabilize it) is an electrophilic reducing agent. In chemistry lingo, electrophilic means it LOVES to hang out with electron-rich partners that can donate electron density.
Carboxylic acids have a uniquely electron-rich oxygen environment that calls out to borane’s electrophilicity. The lone pairs on the acid’s oxygen atoms form a strong interaction with BH3, setting off the reduction process. Ketones, while also carbonyls, don’t present nearly as rich an electron environment because they lack the acidic OH group’s participation.
This fundamental difference explains why borane “picks” the carboxylic acid as its favorite dance partner at the molecular disco.
Selectivity: Fine-Tuning Borane’s Reaction Course
Now, the real magic lies in how chemists control the reaction to keep borane loyal to carboxylic acids. It turns out that the selectivity is all about quantity and timing.
- If you add just the right amount of BH3•THF, it will rush to reduce the carboxylic acid and politely ignore the ketone for the most part.
- However, if you throw in an excess of borane, suddenly even the ketone gets a visit from the electrophilic boss and reduction proceeds there too.
This means you can effectively “skip” ketone reduction if you keep the borane levels in check, a neat trick for synthetic chemists looking to selectively reduce molecules with mixed functional groups.
The chemistry behind this stems from the fact that ketones, although less nucleophilic than acids, will still react if enough reducing agent lingers in the reaction flask. Controlling borane equivalents is key to maintaining precision.
Why Don’t Ketones Get Reduced More Easily by BH3?
Ketones, by nature, have a partial positive charge on the carbonyl carbon and are electrophilic sites themselves. However, borane prefers reacting with nucleophilic sites—parts of the molecule with extra lone pairs like the oxygen in carboxylic acids.
Additionally, the resonance stabilization of the carboxylic acid carbonyl oxygen’s negative charge, combined with its acidic proton, provides a stronger binding site for borane. This makes the carboxylic acid a better partner for reduction by borane than ketones, which don’t have this added acidity or electron density.
A Practical Chemistry Hack: Protecting Ketones to Avoid Unwanted Reduction

Sometimes, chemists want to reduce just the carboxylic acid and keep the ketone in one piece—because who wants the ketone unexpectedly slashed by borane, right?
Here’s a smart workaround:
- Protect the ketone by converting it into an acetal. This is typically done using ethylene glycol and acid (H+).
- Use a stronger reducing agent like LiAlH4 to reduce the securely protected molecule’s carboxylic acid group to an alcohol.
- After the reduction, remove the acetal protecting group using mild acidic conditions (H+/H2O), restoring the original ketone.
This strategy works like a charm and bypasses the tricky issue of borane wanting to reduce everything in sight if not carefully dosed. It’s a neat layered approach that professional organic chemists use regularly to keep their multi-functional molecules happy and intact.
Why Not Use LiAlH4 Directly to Reduce Both?
Good question. LiAlH4 is a powerful hydride donor and indiscriminately cuts through ketones, acids, esters—you name it. So, if you want to spare the ketone and only reduce the acid selectively, BH3•THF is often the milder, more selective choice.
Think of BH3•THF as a tailored suit and LiAlH4 as a bulldozer. Both can do the job but the level of care and precision differs drastically.
Wrapping It Up: The Marvel of Selectivity in Reduction Chemistry
The takeaway? BH3•THF’s electrophilic character and the electronic differences between carboxylic acids and ketones dictate the selectivity seen in reduction reactions.
You get a gentle but efficient reduction of carboxylic acids without beating up your ketones—provided you don’t go wild with the borane amount. In case ketones do need protecting, the acetal strategy is a perfectly reasonable and commonly used method.
Think about your synthetic routes like planning a dinner party. You wouldn’t serve every dish at once, nor would you want your guests (functional groups) to clash. Using borane wisely is like inviting the right crowd and moderating their interactions for the best evening.
Next time you hear “Why does BH3•THF not reduce ketones as well as carboxylic acids?” remember—it’s chemistry’s way of saying, “I like the rich, juicy electron environment of carboxylic acids much better.”
Why does BH3•THF reduce carboxylic acids but not ketones as effectively?
BH3•THF is electrophilic and prefers more electron-rich groups. Carboxylic acids have lone pairs and acidic protons that enhance reaction. Ketones are less reactive toward borane in comparison.
Can BH3•THF reduce ketones if used in excess?
Yes, excess BH3•THF can start reducing ketones as well. Controlling the amount of borane helps selectively reduce the carboxylic acid without affecting the ketone.
Why is borane selective between carboxylic acids and ketones?
Carboxylic acids interact strongly with borane due to their acidic proton and electron density, making reduction faster. Ketones lack these features, slowing their reduction.
What method helps prevent ketone reduction when targeting carboxylic acid?
Protect the ketone by converting it to an acetal using ethylene glycol and acid. Then reduce with LiAlH4, which won’t affect the protected ketone. Finally, remove the acetal.
Why might LiAlH4 be preferred over BH3•THF for reducing carboxylic acids next to ketones?
LiAlH4 is stronger and less selective but can be used after ketone protection. BH3•THF selectively reduces acids but risks ketone reduction if not controlled.




Leave a Comment