Why Is an SN1 Reaction Considered a Two-Step Reaction Despite Additional Intermediates and Transition States?

An SN1 reaction is considered a two-step reaction because its core mechanism involves the formation of a carbocation intermediate after the leaving group departs, followed by nucleophilic attack on this carbocation. While reaction coordinate diagrams may show two intermediates and three transition states, these often include extra protonation or deprotonation steps related to acid-base chemistry, which are not fundamental steps of the SN1 mechanism.
Core Steps of the SN1 Mechanism
- Step 1: The leaving group departs, generating a carbocation intermediate.
- Step 2: The nucleophile attacks the carbocation to form the substitution product.
The carbocation intermediate is the hallmark of the SN1 mechanism. These two steps explain why SN1 reactions are classified as two-step, regardless of additional processes.
Origins of Additional Intermediates and Transition States
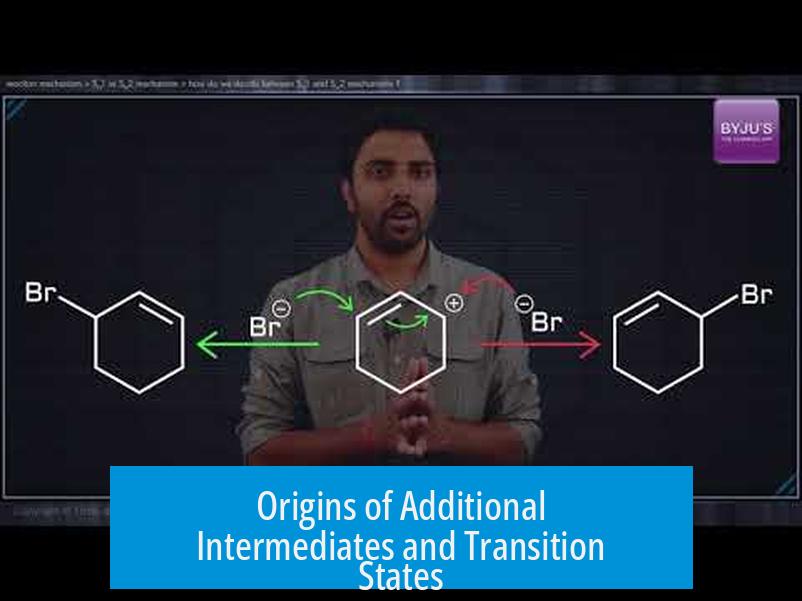
Sometimes, reaction coordinate diagrams depict more intermediates and transition states. These arise mainly from:
- Protonation of the nucleophile or substrate before the main SN1 mechanism.
- Deprotonation after nucleophilic attack when the nucleophile carries an extra proton.
Such acid-base steps create additional species and transition states but are generally very fast and reversible.
Why Acid-Base Steps Are Not Counted as SN1 Steps
Acid-base reactions, including protonation or deprotonation, occur rapidly compared to the slower bond-breaking and bond-forming events defining SN1. The following points clarify this:
- These fast steps do not limit the overall reaction rate.
- They often depend on pH and nucleophile type rather than the substitution mechanism itself.
- They are therefore excluded when counting the fundamental SN1 steps.
Influence of Reaction Conditions and Nucleophile Nature
The presence or absence of additional protonation/deprotonation steps depends on the environment:
- Neutral nucleophiles: May require protonation or deprotonation, adding steps.
- Anionic nucleophiles (e.g., OH−): Follow the classic two-step SN1 with no extra acid-base steps.
- Reaction medium pH: Alters protonation states, affecting intermediates seen.
Summary of Key Points
- SN1 involves carbocation formation and nucleophile attack as fundamental steps.
- Additional intermediates often reflect rapid acid-base reactions, not part of SN1 mechanism.
- Fast protonation/deprotonation steps are typically excluded from SN1 step count.
- Reaction conditions and nucleophile influence the presence of extra steps.
Why is an SN1 Reaction Considered a Two-Step Reaction When the Reaction Coordinate Diagram Shows 2 Intermediates and 3 Transition States?
At first glance, it seems confusing: the SN1 reaction coordinate diagram displays 2 intermediates and 3 transition states, yet textbooks boldly state that SN1 is a two-step process. So, what gives? The key lies in distinguishing the core steps of the SN1 mechanism from the extra acid-base events that often sneak into the full reaction path. Let’s dive deep into this bubbling chemistry pot and unravel the mystery!
Start with the basics. The acronym SN1 stands for nucleophilic substitution (S), unimolecular rate-determining step (N1). The hallmark of SN1 is the formation of a carbocation intermediate after the leaving group leaves. This carbocation is the star of the show—metastable and reactive—waiting eagerly for a nucleophile to attack.
So, the classic two steps are:
- The Leaving Group Departs, forming the Carbocation intermediate.
- The Nucleophile Attacks the Carbocation, producing the product.
This classic storyline earns SN1 its two-step reputation. But things aren’t always that simple. Chemical reactions often wear additional layers of complexity—it’s part of their charm.
Why a Profile May Show 2 Intermediates and 3 Transition States?
If you’ve seen SN1 reaction coordinate diagrams with an extra intermediate and one extra transition state, that usually points to additional protonation or deprotonation events. These are acid-base steps that can show up depending on the nucleophile and reaction environment.
For example, imagine the nucleophile carries an extra proton—like water or an alcohol. After the carbocation is attacked, the product might be protonated. A quick deprotonation follows to give the neutral product. This extra shuffle creates another intermediate: the protonated species.
Thus, the extra intermediate is not a new core step in SN1; it’s an acid-base step tacked on afterward. Similarly, a transition state appears for this deprotonation process.
This means the coordinate diagram reflects both the two classic SN1 steps, plus these fast acid-base adjustments. It’s helpful to remember:
“The final deprotonation is not part of the SN1.” It is conditional and depends on pH or nucleophile identity.
Acid-Base Chemistry: The Swift Guest That Doesn’t Count as a Step
Why don’t these proton shuffles count as separate reaction steps? Because they’re lightning fast. Acid-base reactions proceed much faster than carbocation formation or nucleophilic attack. So fast that chemists practically ignore their time scale when defining SN1 steps.
Think of it this way: you’re timing a meal prep, but the seconds it takes to sprinkle salt on the final dish doesn’t change the overall cooking steps.
In official terms:
“Acid/base reactions (like the deprotonation here) are extremely fast. So, that is basically disregarded as a ‘step.’”
Therefore, even if a reaction coordinate diagram shows more than one intermediate and more than two transition states, the core SN1 remains a two-step mechanism.
Nucleophile and Reaction Conditions Matter a Lot
We can’t just talk SN1 steps without mentioning the role of the nucleophile. Consider this:
- If the nucleophile is hydroxide ion (OH−), the mechanism is a clear-cut two-step SN1 with no extra protonations or deprotonations. You directly get nucleophilic attack on the carbocation without detours.
- If the nucleophile is water, things get more complicated. Water may first protonate or deprotonate before or after the main steps, resulting in extra intermediates on the diagram.
This means chemists often assume a simplified, “idealized” nucleophile to focus on the essential two steps when defining SN1. Variations are just “decorations” added by reaction conditions.
Let’s Put This into a Practical Perspective
Suppose you’re studying an SN1 reaction in the lab—say, solvolysis of tert-butyl chloride in water. You might observe intermediates beyond the carbocation due to solvent interactions and proton transfers. However, when you analyze the rate law and mechanism, the slowest and decisive events remain:
- Departure of Cl− to form tert-butyl carbocation.
- Attack of the water nucleophile on this carbocation.
The proton transfers happen fast and can’t be isolated as fundamental steps dictating the reaction speed or pathway. So, your synthetic report will—and should—call this a two-step SN1 reaction.
Summary: SN1 Is Two Steps, But the Diagram Tells More Stories
| Aspect | Explanation |
|---|---|
| Core SN1 Steps | 1) Leaving group leaves (carbocation forms), 2) nucleophile attacks carbocation. |
| Additional Intermediates | Often protonated/deprotonated species from acid-base reactions, not SN1 steps. |
| Extra Transition States | Represent fast proton transfer events, not rate-limiting SN1 process. |
| Impact on Mechanism Label | Protonation/deprotonation ignored when counting SN1 steps—still considered two steps. |
In other words, an SN1 reaction is considered two-step because only the formation of the carbocation and the nucleophilic attack define the true mechanism. Extra proton juggling happens backstage, making the story seem longer if you only glance at the reaction coordinate.
Now you know why chemists stick with the elegant simplicity of “two steps” even when the reaction coordinate tells a richer tale. This understanding helps avoid confusion when interpreting complex reaction profiles or teaching introductory organic chemistry.
Next time you see a multi-intermediate SN1 diagram, smile and remember: chemistry loves drama, but some acts are just cameo appearances!
Why is an SN1 reaction described as a two-step process despite having multiple intermediates?
The SN1 mechanism centers on two fundamental steps: the leaving group departs to form a carbocation, then the nucleophile attacks it. Extra intermediates often come from acid-base reactions, which are not part of the core SN1 steps.
What causes extra intermediates and transition states to appear in the SN1 reaction diagram?
Protonation or deprotonation events can add intermediates and transition states. These relate to acid-base chemistry, such as protonating the nucleophile or deprotonating after nucleophilic attack, and are not intrinsic SN1 steps.
Are protonation and deprotonation considered actual steps in the SN1 mechanism?
No. These acid-base steps are very fast and usually ignored when defining the SN1 mechanism. They depend on the reaction conditions and nucleophile but don’t change the fundamental two-step nature.
How do nucleophiles affect the number of steps observed in the SN1 reaction?
Nucleophiles that carry a proton may require an additional deprotonation after attack, increasing observed steps. Strong nucleophiles like OH- typically follow the classic two-step SN1 without extra acid-base steps.
Why do reaction coordinate diagrams sometimes show three transition states for an SN1 reaction?
Because diagrams include all molecular events, including fast acid-base changes. These create extra transition states beyond the two main ones related to carbocation formation and nucleophile attack.




Leave a Comment