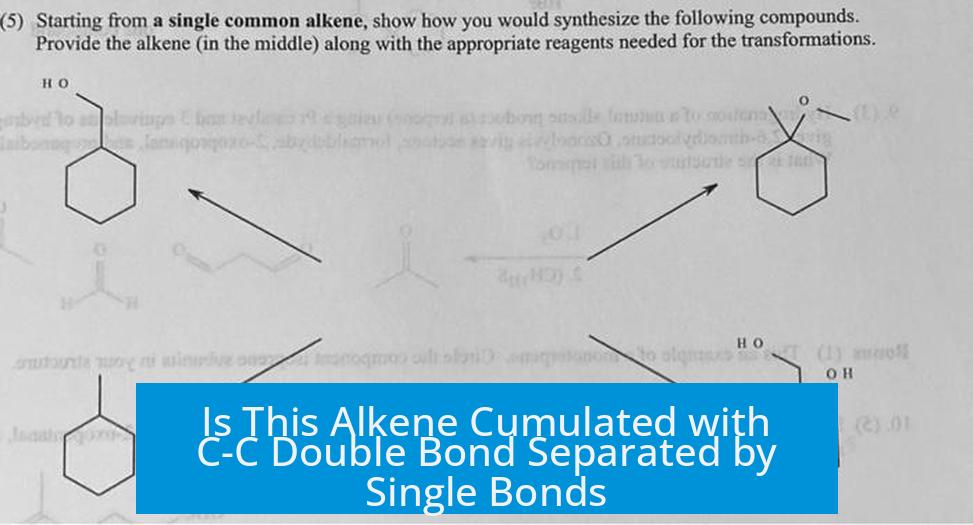
Is the Alkene Considered Cumulated Despite Multiple C-C Single Bonds on One Side?
Yes, the alkene is considered cumulated even if the C=C double bond is adjacent to several C-C single bonds on the opposite side. This is because the central carbon in the double bond is sp hybridized, imposing a linear geometry typical of cumulated double bonds.
Hybridization and Molecular Geometry
The central carbon in such an alkene adopts sp hybridization. This results in a bond angle of roughly 180°, unlike the typical 120° of sp2 carbons found in isolated alkenes.
While this linearity introduces considerable strain if other substituents or bridges impose geometric constraints, it fundamentally classifies the double bonds as cumulated.
Classification as a Cumulene
By definition, cumulated alkenes, or cumulenes, have consecutive double bonds without intervening single bonds. In this case, the positioning around the sp hybridized carbon means the molecule fits the cumulene category structurally.
Even if the alkene has a connected chain of single bonds on one side, the conjugation and hybridization at the double bond maintain its cumulated nature.
Relation to Allenes and Cyclic Structures
This alkene can be viewed as a variant of an allene, where the ends are linked by a -CH2-CH2-CH2- bridge. Such structural restriction introduces strain, but also unique properties such as chirality.
Examples like cyclononallene, a 9-membered cyclic allene, demonstrate possible stable cyclic cumulene structures despite potential strain.
Chirality and Stability
- The molecule exhibits chirality due to the orthogonal arrangement of the π bonds.
- This chirality persists even when the molecule is cyclic or conforms to bridge restraints.
- Though strained, certain cyclic derivatives are isolable and stable enough for study.
Experimental Evidence and Reactivity
Such cumulene-like molecules do exist transiently. They often appear as reactive intermediates in chemical reactions.
Recent research, including Garg’s studies, provides experimental support for the real existence of these strained cumulenes despite their unusual bonding. Garg’s work highlights their detection and characterization.
Key Takeaways
- The central carbon of the alkene is sp hybridized, leading to cumulated double bonds.
- A chain of single bonds on one side does not negate its classification as a cumulene.
- Structural analogs like cyclic allenes exist and can be chiral and stable.
- Such molecules may be highly strained but are real and can be reactive intermediates.
Is the alkene considered cumulated when surrounded by C-C single bonds?
Yes, it is still considered cumulated. The central carbon is sp hybridized, which fits the cumulene definition despite the single bonds on the other side.
How does the sp hybridization affect the molecule’s structure?
Sp hybridization causes the carbon’s bond angle to be 180°, making the molecule linear. This creates significant strain especially in cyclic systems or when connected by bridges.
Can such a strained cumulene-like molecule exist in reality?
Yes. Although strained, these molecules exist as reactive intermediates. Experimental studies, like those by Garg, confirm their transient presence.
What is the relation between this alkene and allenes?
The molecule resembles an allene with a -CH2-CH2-CH2- bridge linking the ends. This influences its geometry and chirality but retains cumulene characteristics.
Does the molecule exhibit chirality and stability?
It does show chirality due to its structure. Related cyclic allenes, such as cyclononallene, demonstrate that certain cyclic cumulenes can be stable.


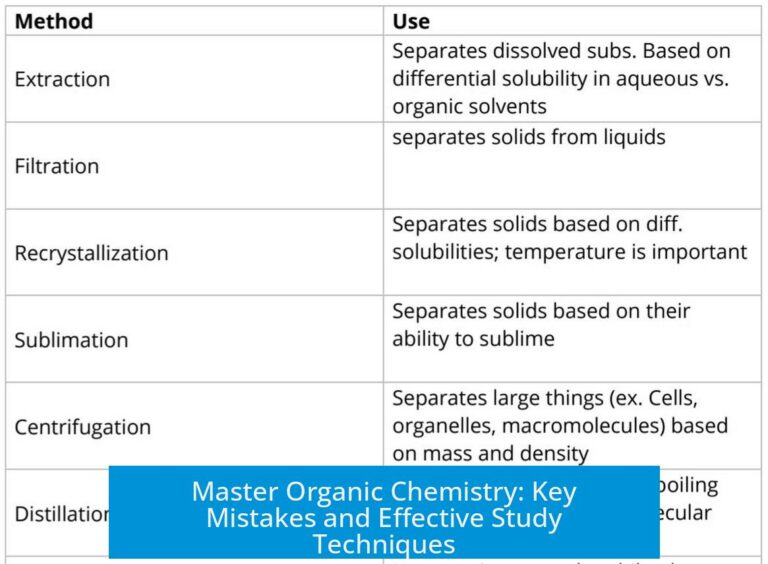

Leave a Comment