Do you ever get confused about electron configuration? If so, you are not alone. Electron configuration is a complex topic that can be difficult to understand. It involves understanding the structure of atoms and how electrons are arranged around them. Knowing the electron configuration of an atom is important in chemistry, physics, and biology. It is also necessary in order to determine the properties of a particular element.
In this blog post, we will discuss what the electron configuration 1s2 2s2 2p6 3s1 means and how to figure out the electron configuration of different elements. We will also go over some examples of electron configurations and how to identify the element associated with a given electron configuration. Finally, we will provide a simple guide to help you understand electron configuration in no time.
Electron configuration is the arrangement of electrons in an atom’s orbital shells. To determine the electron configuration of an atom, you need to know its atomic number. This is the number of protons in the nucleus of an atom and is also equal to the number of electrons that it has. The atomic number of an atom determines its place on the periodic table.
For example, the electron configuration of sodium (Na) is 1s2 2s2 2p6 3s1. This means that the atom of sodium has 11 electrons, with two in the first shell, two in the second shell, six in the third shell, and one in the fourth shell. The super subscripts (2, 2, 6, 1) are the number of electrons in each orbital. Simply add up these numbers, which will give you 11. Now just look for the element on the periodic table with the atomic number of 11.
Similarly, the electron configuration of magnesium (Mg) is 1s2 2s2 2p6 3s2. This means that the atom of magnesium has 12 electrons, with two in the first shell, two in the second shell, six in the third shell, and two in the fourth shell. Again, the super subscripts (2, 2, 6, 2) are the number of electrons in each orbital. Simply add up these numbers, which will give you 12. Now just look for the element on the periodic table with the atomic number of 12.
By understanding the basics of electron configuration, you can easily identify the atomic number of an element from its electron configuration. You can also use this knowledge to identify the element associated with a given electron configuration. With this information, you can start to understand the properties of different elements and their role in chemistry, physics, and biology.
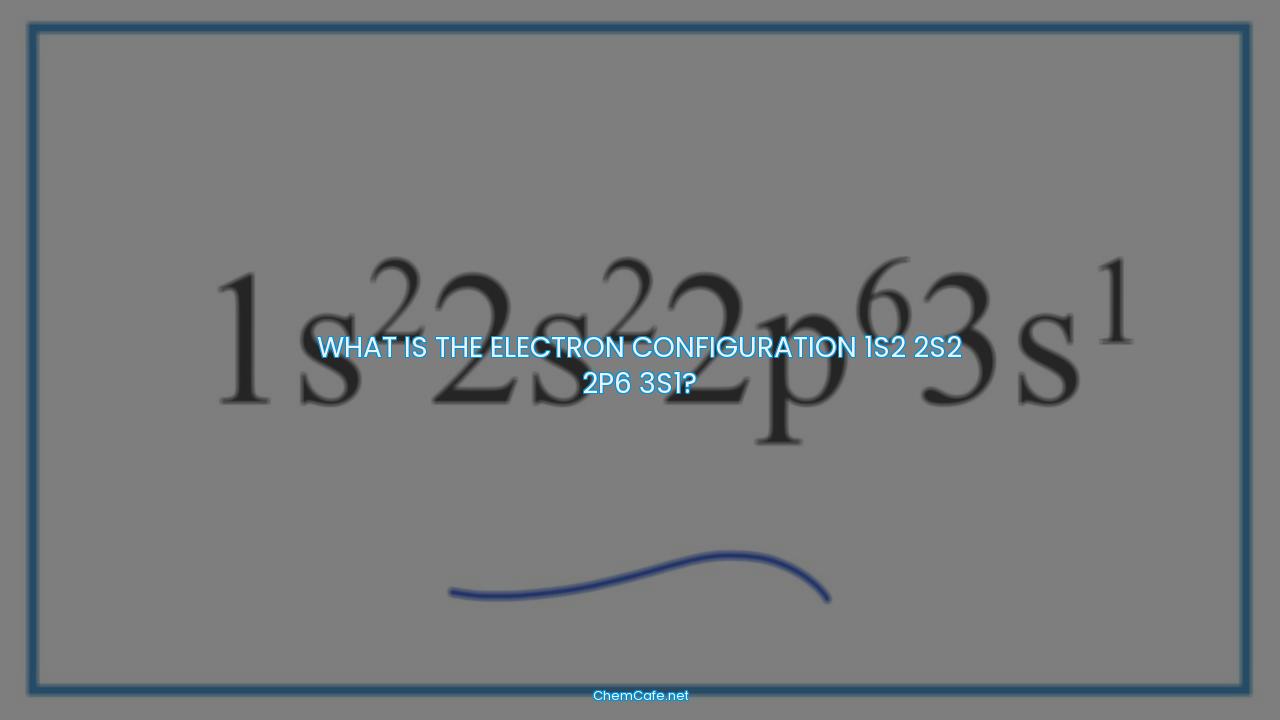
What is the electron configuration 1s2 2s2 2p6 3s1?
The electron configuration of an atom is an important factor in determining its physical and chemical properties. It provides insight into the arrangement of electrons in an atom and can help predict how an atom will interact with other atoms. In this article, we’ll explore the electron configuration of 1s2 2s2 2p6 3s1 and discuss how to determine the atomic number of an element.
What is the Electron Configuration?
The electron configuration of an atom is a representation of the arrangement of electrons in an atom’s outermost shell. It is typically written using the noble gas notation, which uses the symbol of a noble gas to represent the outermost shell of electrons. The electron configuration of an atom is usually written in the form of 1s2 2s2 2p6 3s1, with the number of electrons in each orbital written as a superscript.
What Does 1s2 2s2 2p6 3s1 Mean?
The electron configuration 1s2 2s2 2p6 3s1 refers to an atom with 11 electrons, with two electrons in the 1s orbital, two electrons in the 2s orbital, six electrons in the 2p orbital, and one electron in the 3s orbital. The superscripts (2, 2, 6, 1) represent the number of electrons in each orbital.
How to Determine the Atomic Number?
The number of electrons in an atom can be used to determine the atomic number of the element. Simply add up the number of electrons from the electron configuration, which in this case is 11. Then look for the element on the periodic table with the atomic number of 11. The element is sodium, with the symbol Na.
How Does the Electron Configuration Affect the Properties of an Atom?
The electron configuration of an atom is closely related to its chemical and physical properties. Atoms with different electron configurations can have different chemical reactivities, stability, and forms of bonding. Thus, the electron configuration of an atom can provide insight into how it will interact with other atoms.
The electron configuration 1s2 2s2 2p6 3s1 refers to an atom with 11 electrons, with two electrons in the 1s orbital, two electrons in the 2s orbital, six electrons in the 2p orbital, and one electron in the 3s orbital. This electron configuration is associated with the element sodium, and can provide insight into the chemical and physical properties of an atom. Understanding the electron configuration of an atom is essential for predicting and understanding its behavior.
What is the electron configuration 1s2 2s2 2p6 3s2 3p4?
Electron configuration refers to the arrangement of electrons in an atom or molecule. It is determined by the Aufbau principle, which states that electrons fill orbitals in order of increasing energy. According to this principle, electrons are filled in the following order: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p…
Therefore, the electron configuration 1s2 2s2 2p6 3s2 3p4 reflects that there are two electrons in the 1s orbital, two electrons in the 2s orbital, six electrons in the 2p orbital, two electrons in the 3s orbital, and four electrons in the 3p orbital. This configuration is commonly referred to as the ground state of the atom, meaning that it is the lowest energy state.
What are Electron Configurations?
Electron configurations of atoms follow a standard notation in which all electron-containing atomic subshells (with the number of electrons they hold written in superscript) are placed in a sequence. For example, the electron configuration of sodium is 1s22s22p63s1. This notation is based on the Aufbau principle, which states that electrons fill orbitals in order of increasing energy.
Table of Electron Configurations
When n=4, the subshells correspond to l=0, l=1, l=2, and l=3 and are named the s, p, d, and f subshells, respectively. The maximum number of electrons that can be accommodated by a subshell is given by the formula 2*(2l + 1). Therefore, the s, p, d, and f subshells can accommodate a maximum of 2, 6, 10, and 14 electrons, respectively. All the possible subshells for values of n up to 4 are tabulated below:
| Subshell | n | l | Max number of electrons |
|---|---|---|---|
| s | 1 | 0 | 2 |
| p | 2 | 1 | 6 |
| d | 3 | 2 | 10 |
| f | 4 | 3 | 14 |
Exceptions to the Aufbau Principle
It is important to note that there exist many exceptions to the Aufbau principle such as chromium and copper. In these cases, electrons are filled in orbitals with lower energy before those with higher energy. This is due to the presence of the so-called “inert pair effect”, which is caused by the repulsion between the electrons in the last occupied orbital and those in the next higher orbital.
In conclusion, the electron configuration 1s2 2s2 2p6 3s2 3p4 represents the ground state of an atom, and is determined by the Aufbau principle. There exist exceptions to this principle, such as chromium and copper, where electrons are filled in orbitals with lower energy before those with higher energy. It is important to remember that the maximum number of electrons that can be accommodated by a subshell is given by the formula 2*(2l + 1).
What has the electron configuration 1s2 2s2 2p6 3s2 3p6?
The electron configuration 1s2 2s2 2p6 3s2 3p6 is the electronic structure of an atom of the element Nitrogen (N). This configuration is obtained by the filling of electrons in the atomic orbitals, starting from the lowest energy level. The 1s orbital is the first level and can hold two electrons, 2s and 2p orbitals are the second level and can hold up to 8 electrons, 3s and 3p orbitals are the third level and can hold up to six, and so on.
Do you see how the numbers add up to fourteen? The 1s orbital has two electrons, the 2s and 2p orbitals each have eight electrons (2s2 2p6 = 8), and the 3s and 3p orbitals each have two electrons (3s2 3p6 = 2). Therefore, 1s2 2s2 2p6 3s2 3p6 = 14 electrons.
How many valence electrons does 1s2 2s2 2p6 have?
For the given electron configuration, the valence shell would be the 4th energy level which include the 4s orbital. Thus, it only has two valence electrons since the 4s orbital has 2 electrons.
Is 1s2 2s2 2p6 stable?
Yes, the electron configuration 1s2 2s2 2p6 is very stable. This is because it is the most stable electronic configuration for Nitrogen, which is the element with the atomic number 7.
What are Electron Configurations?
The electron configuration of an element describes how electrons are distributed in its atomic orbitals. Electron configurations of atoms follow a standard notation in which all electron-containing atomic subshells (with the number of electrons they hold written in superscript) are placed in a sequence. For example, the electron configuration of sodium is 1s22s22p63s1.
However, the standard notation often yields lengthy electron configurations (especially for elements having a relatively large atomic number). To make the notation more convenient and understandable, an alternative notation known as the “noble gas core” notation is used. In this notation, the electrons are written in the order of their filling in the atomic orbitals, starting from the lowest energy level. The noble gas element that precedes the element of interest is written in the beginning of the configuration to indicate the core electrons. For example, the electron configuration for chlorine (Cl) can be written as [Ne]3s23p5.
In conclusion, the electron configuration 1s2 2s2 2p6 3s2 3p6 is the electronic structure of an atom of the element Nitrogen (N). It describes the distribution of electrons in the atomic orbitals of N and is a very stable configuration. The valence shell of 1s2 2s2 2p6 includes the 4s orbital and has two valence electrons. Using the noble gas core notation, the configuration can be written as [He]2s2 2p6 3s2 3p6.
What is the electron configuration 1s2 2s2 2p2?
Electron configurations are used to describe how electrons are distributed in the atomic orbitals of an atom. The notation used for electron configurations is called the Aufbau principle, which states that electrons occupy the lowest energy orbitals available first. The electron configuration 1s2 2s2 2p2 is the electron configuration for the element helium.
What are Electron Configurations?
Electron configurations are used to describe how electrons are distributed in the atomic orbitals of an atom. The notation used for electron configurations is called the Aufbau principle, which states that electrons occupy the lowest energy orbitals available first. The electron configuration of an atom is typically represented as a sequence of numbers and letters, indicating the subshells and the number of electrons in each subshell.
How are Electrons Distributed?
The distribution of electrons in an atom is determined by the Aufbau principle. According to this principle, electrons are filled in the following order: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p… This order is illustrated in the diagram below. It is important to note that there exist many exceptions to the Aufbau principle such as chromium and copper.
What is the Electron Configuration for Helium?
The electron configuration for helium is 1s2 2s2 2p2. This means that the helium atom has two electrons in the 1s subshell, two electrons in the 2s subshell, and two electrons in the 2p subshell. Helium is a noble gas, which means that it has a complete valence shell, with all of its electrons in the outermost shell.
What is the Maximum Number of Electrons in a Subshell?
The maximum number of electrons that can be accommodated by a subshell is given by the formula 2*(2l + 1). Therefore, the s, p, d, and f subshells can accommodate a maximum of 2, 6, 10, and 14 electrons, respectively. All the possible subshells for values of n up to 4 are tabulated below.
In conclusion, the electron configuration 1s2 2s2 2p2 is the electron configuration for the element helium. This configuration indicates that helium has two electrons in the 1s subshell, two electrons in the 2s subshell, and two electrons in the 2p subshell. The maximum number of electrons that can be accommodated by a subshell is given by the formula 2*(2l + 1). The Aufbau principle states that electrons occupy the lowest energy orbitals available first. This principle is used to determine the electron configuration of an atom.
What is the electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d7?
Electron configurations are important for understanding the structure and characteristics of elements. They are used to describe the arrangement of electrons in an atom or molecule, and provide a basis for understanding chemical behavior. The electron configuration of an element describes how electrons are distributed in its atomic orbitals.
The electron configuration of an atom is written using subshell labels. These labels contain the shell number (given by the principal quantum number), the subshell name (given by the azimuthal quantum number) and the total number of electrons in the subshell in superscript. For example, if two electrons are filled in the ‘s’ subshell of the first shell, the resulting notation is ‘1s2’.
The Electron Configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d7
The electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d7 is an example of a particularly lengthy electron configuration. It is the electron configuration of the element chromium, which has an atomic number of 24.
The notation 1s2 2s2 2p6 3s2 3p6 4s2 3d7 suggests that the electrons in the atom are distributed in the following manner:
1s2 – two electrons in the 1s subshell
2s2 – two electrons in the 2s subshell
2p6 – six electrons in the 2p subshell
3s2 – two electrons in the 3s subshell
3p6 – six electrons in the 3p subshell
4s2 – two electrons in the 4s subshell
3d7 – seven electrons in the 3d subshell
The electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d7 can also be written as [Ar] 3d7 4s2. This notation is known as the condensed electron configuration, which is a shorthand version of the electron configuration which omits the inner shells.
The electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d7 is just one example of an electron configuration. Different elements have different electron configurations, depending on the number of electrons in the atom. The electron configuration of an element can help explain its chemical properties and reactivity.
Understanding electron configurations is essential for understanding the structure and behavior of atoms and molecules. Being able to interpret and write electron configurations is a key part of understanding the chemistry of elements.
What is the electron configuration 1s2 2s2 2p3?
Do you know what the electron configuration 1s2 2s2 2p3 means? It’s a representation of the electrons in an atom, and it can tell you a lot about the atom. Understanding the electron configuration of an atom can be a bit tricky, but it’s a fundamental part of chemistry.
Atoms are made up of protons and neutrons that make up the nucleus, and electrons that orbit the nucleus in shells. Electron configurations are a way of representing the arrangement of these electrons. They are written in a specific order that follows the Aufbau principle, which states that electrons fill orbitals of lower energy first.
The electron configuration 1s2 2s2 2p3 is the electron configuration of the element Carbon. Carbon is the sixth element on the periodic table and has an atomic number of 6. This means it has 6 protons, 6 neutrons, and 6 electrons.
The electron configuration 1s2 2s2 2p3 tells us that the Carbon atom has two electrons in its first shell, two electrons in its second shell, and three electrons in its third shell. The 1s2 part of the electron configuration tells us that the first shell has two electrons, and the 2s2 2p3 tells us that the second shell has two electrons in the s orbital and three electrons in the p orbital.
The s and p orbitals are the most common types of atomic orbitals. The s orbital is spherical, and the p orbital is shaped like a dumbbell. The number of electrons in an atom’s s and p orbitals can be determined from its electron configuration.
In the electron configuration 1s2 2s2 2p3, the 1s orbital has two electrons, the 2s orbital has two electrons, and the 2p orbital has three electrons. This tells us that Carbon has two electrons in the s orbital, and three electrons in the p orbital.
The number of electrons in an atom’s s and p orbitals can tell us a lot about its behavior. For example, Carbon has two valence electrons, which means it is able to form two chemical bonds. This is why Carbon is such an important element in organic chemistry.
The electron configuration 1s2 2s2 2p3 is just one example of how electrons are arranged in atoms. Every element on the periodic table will have a different electron configuration, and understanding these configurations can give us valuable insights into the behavior of atoms.


Leave a Comment