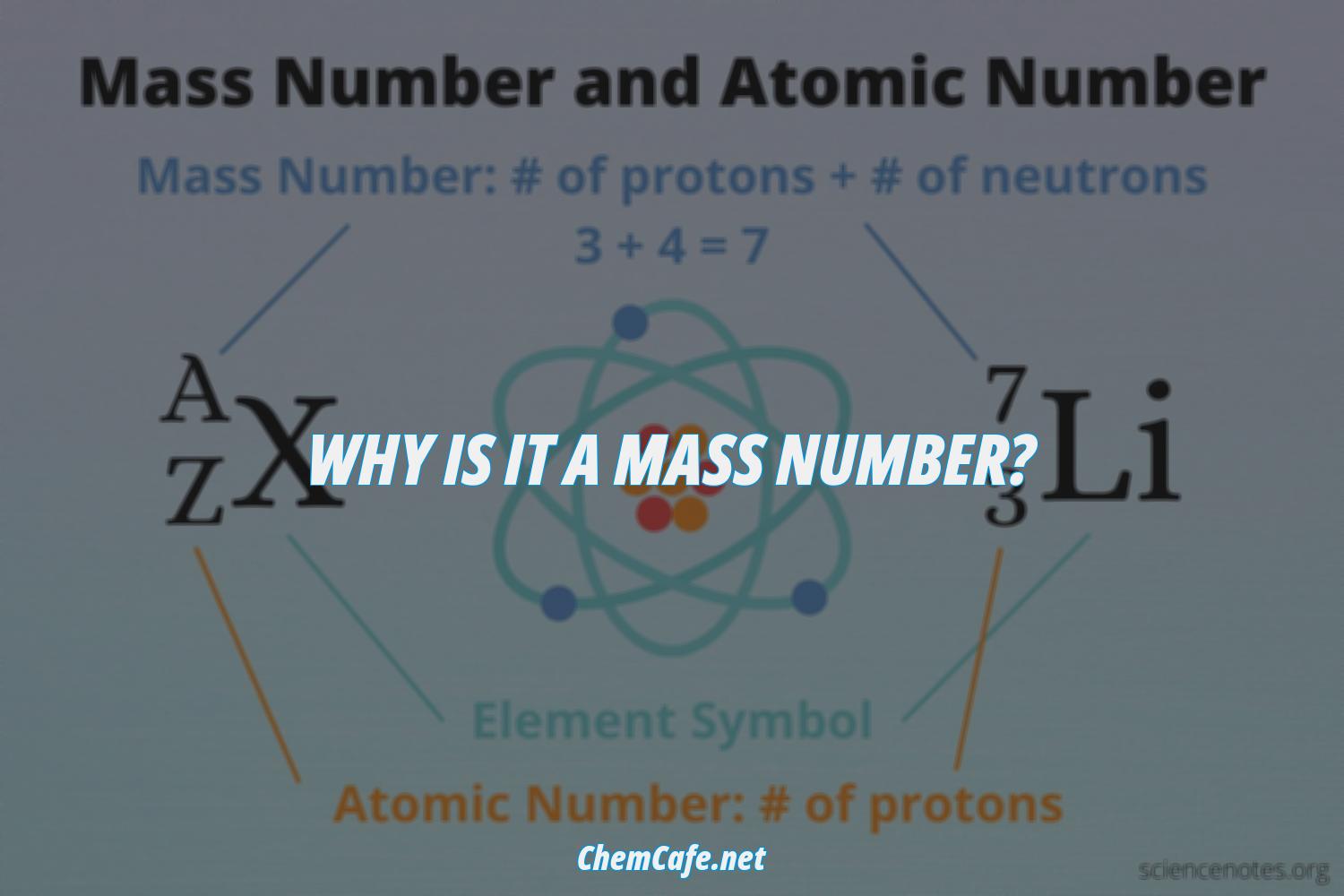
Mass number is an important concept in chemistry, as it helps to identify the number of protons and neutrons in an atom or molecule. The mass number is written as a superscript on the left side of the symbol of the element, and it is always a whole number. It cannot be a fraction.
Mass number is used to indicate the total number of protons and neutrons present in an atom or molecule. It is calculated by adding the atomic number, which is the number of protons, to the number of neutrons. This number is important because it helps to differentiate between different isotopes of an element. For example, He-4 has a mass number 4 and atomic number 2. However, it exists in more variants as He-2, He-3, He-5, He-6, He-7, He-8, He-9, He-10. These variants have the same atomic number but a different mass number.
Mass number is also a useful tool for measuring the mass of an atom or molecule. The mass of an atom is mostly ascribed to its nucleus, which consists of protons and neutrons. The total number of protons and neutrons is called the mass number, and it approximates to the mass of the atom or molecule. It is important to note that both the atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). The sum of the mass number and the atomic number for an atom (A-Z) corresponds to the total number of subatomic particles present in the atom.
In conclusion, mass number is an essential concept in chemistry and is used to identify the number of protons and neutrons in an atom or molecule. It is written as a superscript on the left side of the symbol of the element, and it is always a whole number. It is used to indicate the total number of protons and neutrons present in an atom or molecule, and it is also a useful tool for measuring the mass of an atom or molecule.
Why is it a mass number?
The mass number is an important part of understanding the structure and composition of atoms. It is written as a superscript on the left side of the symbol of the element. Mass number is the sum of the number of protons and neutrons in an atom or molecule. The mass number for all the elements is always a whole number and cannot be a fraction.
Understanding the Mass Number
The mass number is the total number of protons and neutrons in an atom or molecule. This number is calculated by adding the number of protons and neutrons together. The mass number is written as a superscript on the left side of the element symbol. For example, the mass number of helium is 4; this means that the helium atom has 4 protons and 4 neutrons.
Variations of Mass Number
Though, He-4 has a mass number 4 and atomic number 2. However, it exists in more variants as He-2, He-3, He-5, He-6, He-7, He-8, He-9, He-10. These variants have the same atomic number but a different mass number. This is because they contain different numbers of neutrons. The mass number of an atom is determined by its number of protons and neutrons.
Importance of Mass Number
The mass number is important in understanding the structure and composition of atoms. It is used to determine the number of protons and neutrons in an atom. It also helps to identify the isotope of an element. By knowing the mass number, scientists can differentiate between different isotopes of the same element.
Atomic Number and Mass Number
Atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). The sum of the mass number and the atomic number for an atom (A-Z) corresponds to the total number of subatomic particles present in the atom. The mass number reports the mass of the atom’s nucleus in atomic mass units (amu). This is why it is called a mass number.
In summary, the mass number is the sum of the number of protons and neutrons in an atom or molecule. It is written as a superscript on the left side of the element symbol. It is important in understanding the structure and composition of atoms and used to identify isotopes of the same element. It is always a whole number and cannot be a fraction. The sum of the mass number and the atomic number for an atom corresponds to the total number of subatomic particles present in the atom.
What is it called mass number?
The mass number is an important and essential concept in chemistry. It is the total number of protons and neutrons in an atom’s nucleus, and it is often denoted using the capital letter A. It is a whole number that can be used to identify the element, and it is an important concept in understanding isotopes. In this article, we will explore what mass number is, its characteristics, and its importance in understanding isotopes.
What is the Mass Number?
The mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. In other words, it is the sum of the number of nucleons in an atom. Mass number is often denoted using a capital letter A. Contrast this with the atomic number, which is simply the number of protons.
Characteristics of Mass Number
The mass number is written as a superscript on the left side of the symbol of the element. For example, the symbol for helium is He and the mass number is 4. The mass number for all the elements is always a whole number. It cannot be a fraction. Though, He-4 has a mass number 4 and atomic number 2. However, it exists in more variants as He-2, He-3, He-5, He-6, He-7, He-8, He-9, He-10. These variants have the same atomic number but a different mass number.
Atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). The sum of the mass number and the atomic number for an atom (A-Z) corresponds to the total number of subatomic particles present in the atom. The mass number reports the mass of the atom’s nucleus in atomic mass units (amu).
Importance of Mass Number
The mass number is an important concept for understanding isotopes. Isotopes are atoms of the same element that have different mass numbers. Isotopes have the same atomic number but different mass numbers because their nuclei contain different numbers of neutrons. For example, hydrogen has three isotopes: hydrogen-1 (H-1), hydrogen-2 (H-2), and hydrogen-3 (H-3). The mass number of these isotopes are 1, 2, and 3 respectively.
The mass number is also important for calculating the average atomic mass of an element. This is because the average atomic mass is the weighted average of the masses of all its naturally occurring isotopes. For example, the average atomic mass of carbon is 12.0107 amu because it has two isotopes, carbon-12 and carbon-13, with masses of 12 and 13 amu respectively.
In conclusion, the mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. It is written as a superscript on the left side of the symbol of the element, and it is always a whole number that cannot be a fraction. The mass number is an important concept for understanding isotopes, as isotopes have the same atomic number but different mass numbers. It is also important for calculating the average atomic mass of an element.
What is mass number called?
The mass number of an atom is an important factor in determining its properties and behavior. Mass number, also known as atomic mass, is the total number of protons and neutrons in an atom. It is denoted using the letter A and is an integer (whole number).
The mass of an atom is mostly ascribed to a nucleus, which consists of protons and neutrons. The total number of protons and neutrons is called the mass number. Therefore, the mass number is an approximate mass of a given isotope of an element.
It is important to differentiate the mass number from the atomic number, which is the total number of protons in a particular atom. When writing out descriptions of a particular atom, sometimes the mass number is included.
What is the relationship between mass number and atomic number?
The mass number, A, is equal to the sum of the number of protons and neutrons in an atom. On the other hand, the atomic number, Z, is the total number of protons in a particular atom. Therefore, the mass number is always greater than the atomic number.
For example, the mass number of oxygen-16 (O-16) is 16 and its atomic number is 8. This means that the O-16 atom has 8 protons and 8 neutrons in its nucleus.
What is the significance of the mass number?
The mass number is important for determining the properties and behavior of an atom. It helps us understand how an atom will interact with other atoms, and how it will react in various conditions.
The mass number is also important for understanding nuclear physics. It helps us calculate the energy of a nucleus, as well as its stability.
What is the importance of isotopes?
Atoms of the same element can have different mass numbers, depending on the number of neutrons they contain. These atoms are called isotopes and they play an important role in nuclear physics.
Isotopes can be used to study the behavior of various elements in different environments. They can also be used to understand how certain atoms interact with each other.
The mass number of an atom is an important factor in determining its properties and behavior. It is the total number of protons and neutrons in an atom and is denoted using the letter A. The mass number is always greater than the atomic number, which is the total number of protons in a particular atom.
Atoms of the same element can have different mass numbers, depending on the number of neutrons they contain. These atoms are called isotopes and they play an important role in nuclear physics. Therefore, the mass number is important for understanding the behavior of various elements in different environments.
What is the mass number of all elements?
The mass number of an element is the sum of the number of protons and neutrons in an atom. It is also known as the nucleon number and is usually written as A, where A stands for the mass number. The mass number is important for distinguishing between different isotopes of the same element.
Who first determined atomic weights for elements?
The first atomic weights were determined by the English chemist John Dalton in 1803. Dalton used a method based on the relative weights of elements in a compound to determine the atomic weights of elements.
What were the original atomic weights based on?
The original atomic weights were based on the relative weights of elements in a compound. Dalton used a method called “chemical equivalents” to determine the atomic weights of elements. This method involved comparing the weights of elements in a compound to the weights of the same elements in a different compound.
Why were calculations based on numbers of protons not valid for determining atomic weights?
Calculations based on numbers of protons are not valid for determining atomic weights because protons are much lighter than neutrons. The mass number of an element is the sum of the number of protons and neutrons in an atom. Since neutrons are much heavier than protons, the mass number is much larger than the atomic number.
A tin atom has an atomic number of 50 and a mass number of 118. How many neutrons are present in this atom?
The number of neutrons in a tin atom with an atomic number of 50 and a mass number of 118 can be calculated by subtracting the atomic number from the mass number. In this case, the number of neutrons is 118 – 50 = 68.
In conclusion, the mass number of an element is the sum of the number of protons and neutrons in an atom. The atomic weight of an element is approximately equal to its mass number, but can be slightly different due to isotopes. The mass number of a tin atom with an atomic number of 50 and a mass number of 118 is 68.
Why is mass number A?
Atoms are composed of subatomic particles, including protons, neutrons, and electrons. The sum of the mass number and the atomic number for an atom (A-Z) corresponds to the total number of subatomic particles present in the atom. The mass number, denoted by A, is the total number of protons and neutrons present in the nucleus of an atom.
What is the Mass Number?
The mass number is written as a superscript on the left side of the symbol of the element. It is always a whole number and can never be a fraction. It is the same for all atoms of the same element, though some elements exist in different variants that have the same atomic number, but a different mass number. For example, Helium exists in variants He-2, He-3, He-4, He-5, He-6, He-7, He-8, He-9, and He-10.
The mass number is defined as the total number of protons and neutrons in an atom. It can be calculated by adding the number of neutrons and the number of protons (atomic number) together. Mass number = atomic number + number of neutrons
Why is the mass number important?
The mass number is an important part of the periodic table. It helps us understand the properties of the element, as well as its chemical and physical properties. It can also be used to calculate the average mass of atoms of an element.
The mass number is also important in the study of nuclear reactions, as the mass number is the sum of the number of protons and neutrons in the nucleus. This is important in understanding the process of radioactive decay, as well as the energy released in nuclear fission and fusion reactions.
Examples of Mass Number
Table (PageIndex{1}) below shows data from the first six elements of the periodic table.
| Element | Symbol | Atomic Number | Mass Number |
|---|---|---|---|
| Hydrogen | H | 1 | 1 |
| Helium | He | 2 | 4 |
| Lithium | Li | 3 | 7 |
| Beryllium | Be | 4 | 9 |
| Boron | B | 5 | 11 |
| Carbon | C | 6 | 12 |
The mass number for each element is the sum of the atomic number and the number of neutrons. For example, the mass number of Carbon is 12, as it has an atomic number of 6 and 6 neutrons.
The mass number is also used to calculate the average mass of an element. This is done by taking the atomic mass of each isotope of the element and weighting them according to their abundance on Earth. For example, the average mass of Carbon is 12.011 amu, as Carbon has two isotopes, Carbon-12 and Carbon-13, with masses of 12 and 13 amu, respectively.
The mass number is an important part of the periodic table and is used to identify elements and understand their properties. It is also used to calculate the average mass of an element and to understand nuclear reactions.
The mass number is always a whole number and is written as a superscript on the left side of the element symbol. The mass number is the sum of the number of protons and neutrons in the nucleus of an atom, and can be calculated by adding the atomic number and the number of neutrons.
Why is mass number denoted by a?
Mass number is an important concept in atomic physics, as it helps to identify and distinguish different isotopes of the same element. It is denoted by the letter ‘A’, and is defined as the sum of the number of protons and neutrons in an atom. It is often referred to as the atomic mass.
What is Mass Number?
Mass number is the total number of protons and neutrons in an atom, and is represented by the letter ‘A’. It is equal to the sum of the number of protons and neutrons in an atom, and is often referred to as the atomic mass. The mass number is an integer, and as such it is always a whole number. It is important to note that the number of electrons in an atom is not included in the mass number.
Atomic Number and Mass Number
The atomic number is the number of protons in an atom, and is represented by the letter ‘Z’. The atomic number is always a whole number, as it is obtained by counting individual protons. The sum of the mass number and the atomic number (A-Z) for an atom corresponds to the total number of subatomic particles present in the atom.
Importance of Mass Number
Mass number is an important concept in atomic physics, as it helps to identify and distinguish different isotopes of the same element. Isotopes are atoms of the same element that have different numbers of neutrons, and therefore have different mass numbers. Mass number is also important in nuclear physics, as it helps to identify different types of nuclei, and to calculate their masses.
Experimental Data
Experimental data showed that the vast majority of the mass of an atom is concentrated in its nucleus, which is composed of protons and neutrons. The mass number is defined as the total number of protons and neutrons in an atom, and is therefore an important indicator of the mass of an atom. Consider the table below, which shows data from the first six elements of the periodic table.
In conclusion, mass number is an important concept in atomic and nuclear physics, as it helps to identify different isotopes of the same element, and to calculate the masses of nuclei. It is represented by the letter ‘A’, and is equal to the sum of the number of protons and neutrons in an atom. Mass number is always a whole number, as it is obtained by counting whole objects (protons, neutrons, and electrons). Experimental data showed that the mass number is an important indicator of the mass of an atom, and is therefore an important concept in understanding atomic structure.




Leave a Comment