Electron Configurations are an important tool used to understand the properties of elements and predict their behavior. They are the way in which electrons are distributed in an atom’s atomic orbitals, and this notation was developed shortly after the Bohr model of the atom was presented by Ernest Rutherford and Niels Bohr in the year 1913.
Electron Configurations are useful for a variety of tasks, such as determining the valency of an element, predicting the properties of a group of elements, and interpreting atomic spectra. Valency is the number of electrons an element needs to gain or lose in order to be stable, and this information is critical for understanding the bonding of atoms.
Groups of elements with similar electron configurations tend to exhibit similar properties, such as reactivity or color. This means that electron configurations can be used to predict the properties of a group of elements, and it also helps to explain why certain elements are often found together in nature.
Atomic spectra is the light that is emitted when an atom is excited by energy. By examining the spectra, scientists can identify elements and their electron configurations. This information is extremely important for understanding the structure of molecules and compounds, which are the basis of all matter.
So, if you’re looking to answer the question, “Which element has the following electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 quizlet?”, you’ve come to the right place! With the information provided in this blog post, you should be able to determine the element with the given electron configuration.
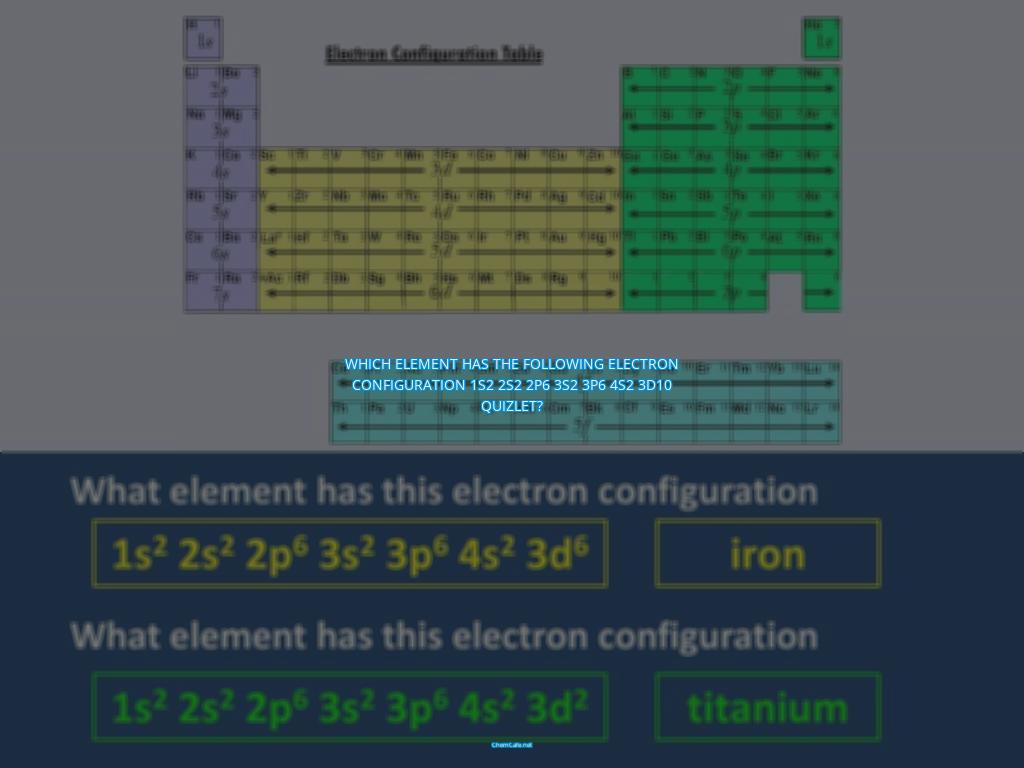
Which element has the following electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 quizlet?
Electron configurations are an important tool used in chemistry to determine the properties of an element. In this article, we’ll discuss what electron configurations are and how they are used to determine the valency of an element, predict the properties of a group of elements, and interpret atomic spectra.
What are Electron Configurations?
Electron configurations are the way electrons are distributed in an atom’s atomic orbitals. This notation for the distribution of electrons in the atomic orbitals of atoms came into practice shortly after the Bohr model of the atom was presented by Ernest Rutherford and Niels Bohr in the year 1913.
The standard notation for electron configurations is quite simple. All electron-containing atomic subshells (with the number of electrons they hold written in superscript) are placed in a sequence. For example, the electron configuration of sodium is 1s22s22p63s1.
What is the Electron Configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10?
The electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 is the configuration for the element chromium (Cr). Chromium is a transition metal in Group 6 of the Periodic Table and has an atomic number of 24. Chromium’s electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1.
What are Electron Configurations Used For?
Electron configurations are useful for a variety of tasks in chemistry. They can be used to determine the valency of an element, predict the properties of a group of elements (elements with similar electron configurations tend to exhibit similar properties), and interpret atomic spectra.
Determining Valency
The valency of an element is the number of bonds it can form with other atoms. This is determined by the number of unpaired electrons in the outermost shell of an atom. Unpaired electrons are electrons that are not shared with other atoms.
For example, the electron configuration of sodium is 1s2 2s2 2p6 3s1. The outermost shell is the 3s shell, which has one unpaired electron. Therefore, sodium has a valency of 1.
Predicting Properties of Elements
Electron configurations can also be used to predict the properties of a group of elements. Elements with similar electron configurations tend to have similar properties, such as reactivity and solubility.
For example, elements in Group 6 of the Periodic Table (chromium, molybdenum, and tungsten) have similar electron configurations and therefore tend to have similar properties.
Interpreting Atomic Spectra
Atomic spectra are the unique patterns of light emitted by atoms when they are excited with energy. Electron configurations can be used to interpret these spectra, as the energy levels of electrons in an atom determine the wavelengths of light that are emitted.
By understanding the electron configurations of atoms, scientists can interpret the patterns of light emitted by atoms and gain insight into their properties.
Conclusion
Electron configurations are an important tool used in chemistry to determine the properties of an element. They can be used to determine the valency of an element, predict the properties of a group of elements, and interpret atomic spectra. The electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 is the configuration for the element chromium (Cr). Understanding electron configurations is key to gaining insight into the properties of atoms.
What element has the following electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3?
Electron configurations are an important tool used to predict the properties of an element and its chemical behavior. They are a way of representing the distribution of electrons in an atom’s orbitals, and can be used to identify the element and its valency. The electron configuration of an element is determined by the number of electrons in its atomic orbitals, and is written in a standard notation.
In the case of the electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3, the element is Scandium, with atomic number 21. Scandium is a transition metal, and is the first element in the group of the so-called ‘rare earth’ elements. It is a silvery-white metal that is relatively soft and ductile.
Notation
The electron configuration of an atom is written in a standard notation that uses subshell labels. These labels include the shell number (given by the principal quantum number), the subshell name (given by the azimuthal quantum number) and the total number of electrons in the subshell in superscript. For example, if two electrons are filled in the ‘s’ subshell of the first shell, the notation used is ‘1s2’.
The notation for the electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3 can be broken down as follows:
1s2: This indicates that the first shell (n = 1) has two electrons in its s subshell.
2s2: This indicates that the second shell (n = 2) has two electrons in its s subshell.
2p6: This indicates that the second shell (n = 2) has six electrons in its p subshell.
3s2: This indicates that the third shell (n = 3) has two electrons in its s subshell.
3p6: This indicates that the third shell (n = 3) has six electrons in its p subshell.
4s2: This indicates that the fourth shell (n = 4) has two electrons in its s subshell.
3d10: This indicates that the third shell (n = 3) has ten electrons in its d subshell.
4p3: This indicates that the fourth shell (n = 4) has three electrons in its p subshell.
Usefulness
Electron configurations are useful for determining the valency of an element, predicting the properties of a group of elements (elements with similar electron configurations tend to exhibit similar properties) and interpreting atomic spectra. They are also helpful in understanding the structure of the periodic table, as elements having similar electron configurations are placed in the same group.
In conclusion, the electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3 is the electron configuration of Scandium, with atomic number 21. Electron configurations are an important tool used to predict the properties of an element and its chemical behavior, and are a useful way of representing the distribution of electrons in an atom’s orbitals.
Which element has an electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3?
Electron configurations are essential tools for understanding the structure and properties of atoms and molecules. They provide an organized way of describing the arrangement of electrons in an atom or molecule, and they can be used to predict the behavior of these particles. The electron configuration of an element describes how electrons are distributed in its atomic orbitals.
Notation
In order to represent the electron configuration of an element, a standard notation is used. This notation includes the shell number (given by the principal quantum number), the subshell name (given by the azimuthal quantum number) and the total number of electrons in the subshell in superscript. For example, if two electrons are filled in the ‘s’ subshell of the first shell, the resulting notation is ‘1s2’.
Importance of Electron Configurations
Electron Configurations are important for a variety of reasons, including:
Determining the Valency of an Element: The valency of an element is the number of electrons it can lose, gain or share in chemical reactions. This is determined by the number of electrons in the outermost shell.
Predicting the Properties of a Group of Elements: Elements with similar electron configurations tend to exhibit similar properties.
Interpreting Atomic Spectra: Atomic spectra are used to identify atoms and molecules and to gain information about their electronic structures. Electron configurations help to interpret the spectra.
History of Electron Configurations
The notation for the distribution of electrons in the atomic orbitals of atoms came into practice shortly after the Bohr model of the atom was presented by Ernest Rutherford and Niels Bohr in the year 1913. This model proposed that electrons are arranged in shells, with each shell containing a certain number of orbitals.
The Element with an Electron Configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3
The element with an electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3 is Selenium (Se). This element has an atomic number of 34, which means that it has 34 electrons. The electron configuration of Selenium is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3. It has six valence electrons in its outermost shell and is classified as a nonmetal.
In conclusion, electron configurations provide an organized way of describing the arrangement of electrons in an atom or molecule. Standard notation is used to represent the electron configuration of an element, which includes the shell number, the subshell name and the total number of electrons in the subshell in superscript. Electron configurations are important for determining the valency of an element, predicting the properties of a group of elements, and interpreting atomic spectra. The element with an electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3 is Selenium (Se).
Which of the following elements has the electron configuration 1s2 2s2 2p6 3s2 3p1?
Electron configurations are an important concept in chemistry, as they can tell us how many electrons are in an atom, and how they are arranged in the atom’s orbitals. Knowing the electron configuration of an element allows us to understand its chemical behavior and predict its reactivity with other elements.
What are Electron Configurations?
Electron configurations are the arrangement of electrons in an atom’s orbitals. Electrons are arranged in shells, or energy levels, around the nucleus of the atom. The number of electrons in a given shell is determined by the principal quantum number, n. The number of electrons in a given shell can be calculated by the formula 2n2.
The arrangement of electrons within a shell is determined by the angular momentum quantum number, l. Each shell can contain up to 2l + 1 orbitals, and each orbital can contain up to 2 electrons. The angular momentum quantum number is given by the letter l, and can have a value of 0, 1, 2, 3, or 4.
Table of Content
Electron configurations are usually written using a standard notation, which contains all electron-containing atomic subshells (with the number of electrons in superscript) in a sequence. For example, the electron configuration of sodium is 1s22s22p63s1.
However, the standard notation often yields lengthy electron configurations, especially for elements with a relatively large atomic number. The electron configuration of silicon (atomic number 14) is 1s2 2s2 2p6 3s2 3p2.
Which of the Following Elements Has the Electron Configuration 1s2 2s2 2p6 3s2 3p1?
The correct answer is option E, magnesium (Mg). Atoms of element magnesium (atomic number 12) contain 12 electrons, which are arranged as follows: 1s2 2s2 2p6 3s2.
Option A, sodium (Na), can be neglected. Atoms of element sodium (atomic number 11) contain 11 electrons, which are arranged as follows: 1s2 2s2 2p6 3s1.
Option B, neon (Ne), is incorrect. Atoms of element neon (atomic number 10) contain 10 electrons, which are arranged as follows: 1s2 2s2 2p6.
Option D, aluminum (Al), is incorrect. Atoms of element aluminum (atomic number 13) contain 13 electrons, which are arranged as follows: 1s2 2s2 2p6 3s2 3p1.
In conclusion, the electron configuration of magnesium (Mg) is 1s2 2s2 2p6 3s2, making it the correct answer for this question. Knowing the electron configurations of elements is essential for understanding their chemical behavior and predicting their reactivity with other elements.
Which element has the following electron configuration 1s2 2s2 2p6 3s2 3p6 4s1?
When it comes to understanding the structure of atoms, electron configuration plays an important role. Electron configuration is the arrangement of electrons in an atom or molecule, and it helps to determine the properties of an element. In this article, we’ll discuss the answer to the question: which element has the following electron configuration 1s2 2s2 2p6 3s2 3p6 4s1?
What Are Electron Configurations?
Electron configurations are the way electrons are arranged in an atom or molecule. In order to understand the answer to the question, it is important to first understand what an electron configuration is. Electron configurations are usually written in a standard notation, which includes the number of electrons in each atomic subshell. For example, the electron configuration of sodium is 1s22s22p63s1.
The Aufbau Principle
The Aufbau Principle is a rule that dictates how electrons are placed into orbitals. It states that electrons occupy orbitals with lower energies before occupying higher energy orbitals. The energy of an orbital is calculated by the sum of the principal and the azimuthal quantum numbers.
What Does Electron Configuration Tell Us?
Electron configuration not only helps us to determine the answer to the question, but it also helps us to understand the properties of an element. Electron configurations are useful for:
- Determining the valency of an element. Valency is the number of electrons in the outermost shell of an atom, and it is determined by the electron configuration.
- Predicting the properties of a group of elements. Elements with similar electron configurations tend to exhibit similar properties.
- Interpreting atomic spectra. Atomic spectra is the light emitted by an atom or molecule, and it helps us to understand the structure of the atom.
Answer to the Question
Now that we have a better understanding of electron configuration, we can answer the question: which element has the following electron configuration 1s2 2s2 2p6 3s2 3p6 4s1? The answer is Magnesium (Mg). The electron configuration of Magnesium is 1s2 2s2 2p6 3s2.
Electron configuration plays an important role in determining the properties of an element. By understanding the standard notation for electron configuration and the Aufbau Principle, we can answer the question: which element has the following electron configuration 1s2 2s2 2p6 3s2 3p6 4s1? The answer is Magnesium (Mg). Electron configurations are also useful for determining the valency of an element, predicting the properties of a group of elements, and interpreting atomic spectra.
What element has the electron configuration 1s22s22p63s23p2 quizlet?
Electron configurations are used to describe the distribution of electrons within an atom. They are given in the form of a notation that uses the principal and azimuthal quantum numbers to identify the orbitals and subshells of an atom. The electron configuration of an element describes how electrons are distributed in its atomic orbitals.
For example, the electron configuration of sodium is 1s22s22p63s1. This notation for the distribution of electrons in the atomic orbitals of atoms came into practice shortly after the Bohr model of the atom was presented by Ernest Rutherford and Niels Bohr in the year 1913.
What is the Filling of Atomic Orbitals?
The filling of atomic orbitals is determined by the Aufbau Principle, which states that electrons occupy the orbitals having lower energies before occupying higher energy orbitals. The energy of an orbital is calculated by the sum of the principal and the azimuthal quantum numbers.
The electron configuration of an element can be determined by the number of protons and electrons in the element. For example, the element with atomic number 12, magnesium, has an electron configuration of 1s2 2s2 2p6 3s2. This means that the first two electrons fill the 1s orbital, the next two electrons fill the 2s orbital, and the next six electrons fill the 2p orbitals.
The element with the electron configuration 1s22s22p63s23p2 is fluorine. Fluorine, with an atomic number of 9, has nine electrons in total and its electron configuration reflects that. The first two electrons fill the 1s orbital, the next two electrons fill the 2s orbital, the next six electrons fill the 2p orbitals, and the ninth electron fills the 3s orbital.
What are the Uses of Electron Configurations?
Electron configurations are useful for determining the valency of an element, predicting the properties of a group of elements, and interpreting atomic spectra. Knowing the electron configurations of elements allows for the prediction of their properties and behavior. Elements with similar electron configurations tend to exhibit similar properties.
In addition, electron configurations are useful for interpreting atomic spectra. By understanding the energy levels of electrons in an atom, the energies of the emitted and absorbed photons can be predicted. This makes it possible to identify the element from the spectrum of light it emits.
Overall, electron configurations are a useful tool for understanding the structure of atoms and predicting their properties. Knowing the electron configuration of any element, such as fluorine with its electron configuration 1s22s22p63s23p2, can be an invaluable asset in the study of chemistry.



Leave a Comment