Why is This RNA Oligo Making a Weird Donut Shape in My Urea Gel?

The donut shape in the RNA oligo band on a urea gel usually results from overloading the sample and staining artifacts, which cause uneven visualization and concentration-dependent structural effects. This phenomenon is common when high RNA concentrations induce self-structures or uneven dye binding during electrophoresis.
1. Sample Overloading
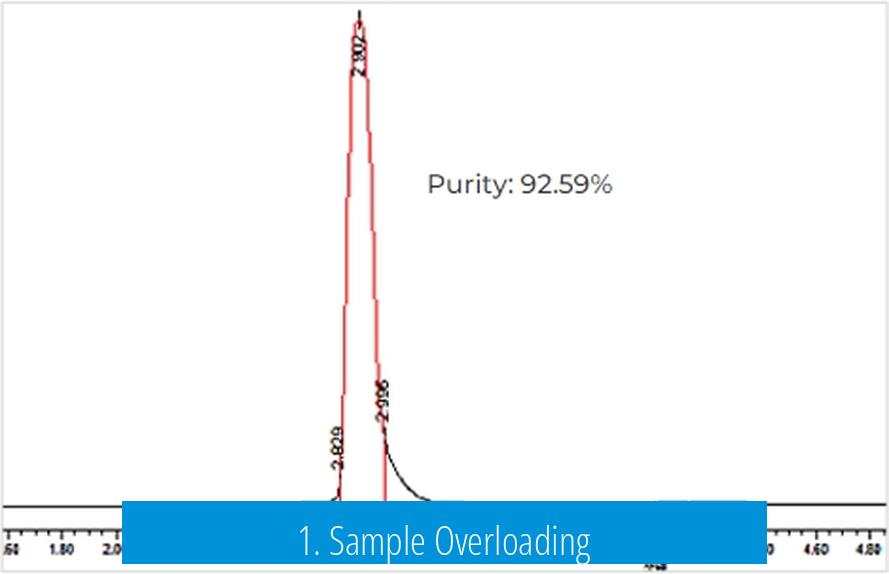
One primary cause of the ring or donut-shaped band is the excessive amount of RNA loaded into the gel well. Highly concentrated RNA causes the center of the band to saturate the imaging sensor. This saturation creates a hollow appearance, with the RNA accumulating and visualizing strongly on the edges but not in the middle.
- Recommended maximum loading: 100 nM concentration or 2 pmoles per well.
- Loading above this threshold often distorts band shape and hinders accurate quantification.
2. Staining and Visualization Effect
The staining method for RNA in urea gels impacts band morphology. Some dyes bind only to single-stranded RNA regions, leaving internal parts less stained if RNA forms transient structures or partial double strands. This pattern yields the typical white center and darker edges, resembling a donut.
Staining consistency can vary depending on the dye type and exposure time. Overstaining can exaggerate these features, while understaining may obscure them. Users often observe these irregular shapes without functional loss of the RNA.
3. RNA Self-Hybridization and Secondary Structures
At high RNA concentrations during electrophoresis, oligonucleotides may self-hybridize or fold into secondary structures. While urea gels are denaturing, certain strong interactions might persist transiently, causing uneven dye uptake.
This can alter the migration speed within the band center differently than at the edges, contributing to donut formation in gel images.
4. Gel Running Conditions
Uneven heating of the gel during the run can slightly modify RNA migration and band appearance. Though less common, gel temperature gradients create artifacts and irregular band shapes.
Monitoring and maintaining consistent electrophoresis temperatures help minimize this effect.
Summary of Key Points
- Excessive RNA loading creates sensor saturation and donut-shaped bands.
- Staining dyes differentially visualize RNA, affecting band morphology.
- High RNA concentration may induce transient self-hybridization, changing dye efficiency.
- Uneven gel temperatures can also influence band appearance.
Why Is This RNA Oligo Making a Weird Donut Shape in My Urea Gel?
If your RNA oligo is showing a funky donut shape in your urea gel, the culprit might be overloading combined with staining quirks and subtle molecular antics—like self-hybridization—turning your gel run into an unexpected art piece. Let’s unpick this molecular mystery with straightforward insights, real-world tips, and a pinch of humor.
Picture this: You run your RNA oligos on a 15% precast urea gel, expecting neat, sharp bands. But, surprise! One oligo forms a donut-shaped blob while the other behaves perfectly. You measured concentrations with a Qubit, cleaned wells, heat-denatured samples, and used RNA dye. Still, that donut stares back.
What’s going on here? Is your oligo trying to make a snack instead of migrating properly? Let’s break down the main suspects behind this phenomenon.
The Usual Suspect: Overloading the Gel
First, ask yourself: Did you load too much RNA? Overloading is the classic reason for weird gel bands. Think of it as cramming too many people into a tiny elevator—things get crowded and messy. In electrophoresis, loading more than 2 pmoles or about 100 nanomolar concentration can saturate your gel and sensor.
One thoughtful researcher pointed out, “Looks like saturation in your sensor due to running a really high concentration.” It’s a solid reminder that more is not always better. The overload can cause the RNA to accumulate unevenly, making the stain pick up differently across the blob. This can produce the characteristic donut shape where the center looks faint or blank, surrounded by a brighter ring.
Why Does the Donut Appear? The Staining Efficiency Mystery
Next, let’s talk stain. That unusual donut could be the result of how the stain interacts with your RNA.
One user shared their experience: “The RNA is mostly the white part of the blob, for some reason only the outer part stains.” This happens because the stain can’t penetrate dense RNA aggregates well, especially in overloaded samples. The outer ring stains well, while the densely packed core remains faint, giving you that hollow donut effect.
Think of staining like soaking a sponge: thick clumps repel the dye inside, so only the surface gets color.
Pro tip: Consider how you stain your gel. Different dyes have varied penetration efficiency, and over-staining can exaggerate artifacts. A gentle, optimized staining protocol can reduce these effects.
Could the Gel Temperature Be Sneaky?
Another angle to consider is the gel’s temperature during electrophoresis. Though not the most common cause, warming gels can mess with RNA migration and band shapes.
Someone chimed in: “If it heats up significantly during the run this may result in strange banding patterns.”
Heating can alter the gel matrix or your oligo’s conformation, influencing migration. Keeping the gel cool during runs (on ice or with a cooling system) can prevent surprises. If you’ve run your gel for a long time or at high voltage, remember temperature can be an underrated troublemaker.
RNA Folding and Self-Hybridization—The Hidden Culpit
One more clue lies in the nature of your RNA oligo. At high concentrations, RNA molecules might self-hybridize, creating double-stranded (ds) regions inside what should be single-stranded (ss) RNA samples.
This matters because stains often bind dsRNA differently—or less efficiently—compared to ssRNA. As a result, if the center of your oligo band harbors self-hybridized RNA, it might stain poorly, leading again to the donut shape.
Someone asked, “Could you be getting self-hybridization at high concentrations that leads to reduced efficiency of your stain for the dsRNA substrate?” It’s a savvy question and highlights that RNA structure affects how your gel ultimately looks.
If you suspect this, try running the gel with freshly denatured samples or lower RNA concentration. Adding formamide or a stronger denaturing step might reduce self-hybridization and yield clearer bands.
Putting It All Together: What You Can Do Right Now
- Check your loading amounts. Keep it under about 2 pmoles (or below 100 nM concentration) to avoid saturation that creates blobs or donuts.
- Re-examine your staining method. Use gentle staining, adjust stain time, or try alternative dyes to see if the artifact persists.
- Ensure gel temperature stays low. Run gels in a cooled environment or at lower voltage to avoid heat-induced distortion.
- Consider RNA structure. Freshly denature your RNA oligos before loading, and consider additives that discourage self-hybridization.
Don’t forget, as frustrating as donuts on gels can be, these odd shapes don’t always mean your RNA is ruined. Many researchers see this “donut blob” but still manage to purify and use that RNA successfully.
One researcher shared their perspective: “I’ve always succeeded in purifying and using them as if they’re just fine!” So, while the donut is weird to look at, it’s not necessarily a disaster.
Why Does This Matter? The Take-Home Lesson
Understanding the cause of unusual gel shapes matters for anyone working with RNA.
- It saves you from pointless troubleshooting and expense.
- It ensures consistent data for your experiments.
- It helps you trust your results without puzzling over weird images.
So next time you see that mysterious donut on your urea gel, don’t panic. Use this guide to decode the cause and fix your run. In essence, that silly donut is a molecular message—a hint to dial back your loading, check stains, mind temperature, and remember RNA loves to fold and play tricks.
Parting Thoughts: Donuts Aren’t Just for Breakfast
Tackling RNA gel puzzles proves science is as much an art as a precise technique. Sometimes your oligo wants to be a doughnut. When it happens, laugh a little, troubleshoot smartly, and share your story. The community of scientists is full of helpful voices ready to say, “Ah yes, you’re making a donut from scratch.”
Feeling stuck? Reach out on forums. Many have been there and seen that quirky gel shape before turning it around successfully.
After all, science is better together. And who knows? Your weird donut might just be the beginning of a tasty new protocol!
Why does my RNA oligo form a donut shape in a urea gel?
This shape often results from overloading the gel. High RNA concentration saturates the signal, causing uneven staining and the donut appearance.
Can staining methods cause the donut pattern in RNA gels?
Yes, some stains bind only to the outer parts of the RNA, creating an uneven signal. This makes the center of the band appear lighter or empty.
Could the gel running temperature affect the RNA band shape?
If the gel heats up during the run, it can cause unusual banding patterns. Temperature shifts may alter RNA structure or stain behavior, influencing the shape.
Is self-hybridization responsible for the weird donut pattern?
At high RNA concentrations, self-hybridization can occur. This alters stain binding, especially in the center, causing reduced staining and a hollow band.
How can I prevent the donut shape in future RNA gel runs?
Use lower RNA amounts to avoid saturation. Verify staining techniques and maintain consistent gel temperatures during electrophoresis to ensure uniform band shapes.




Leave a Comment