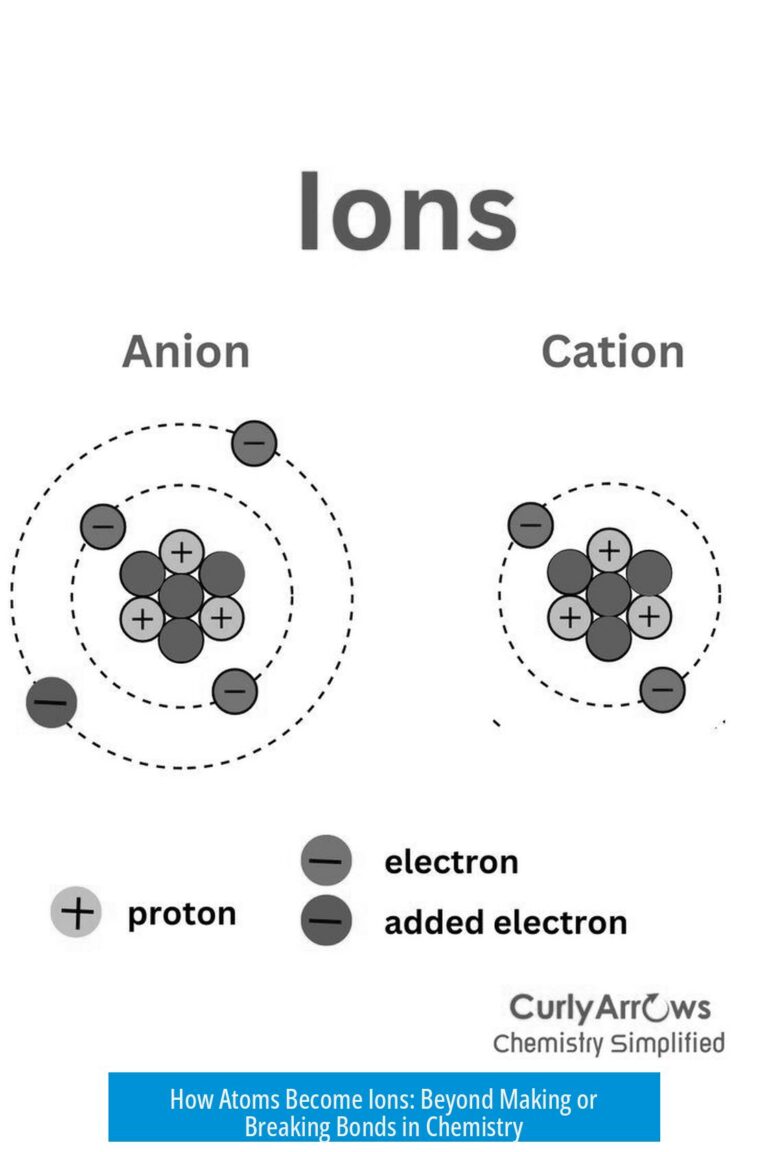
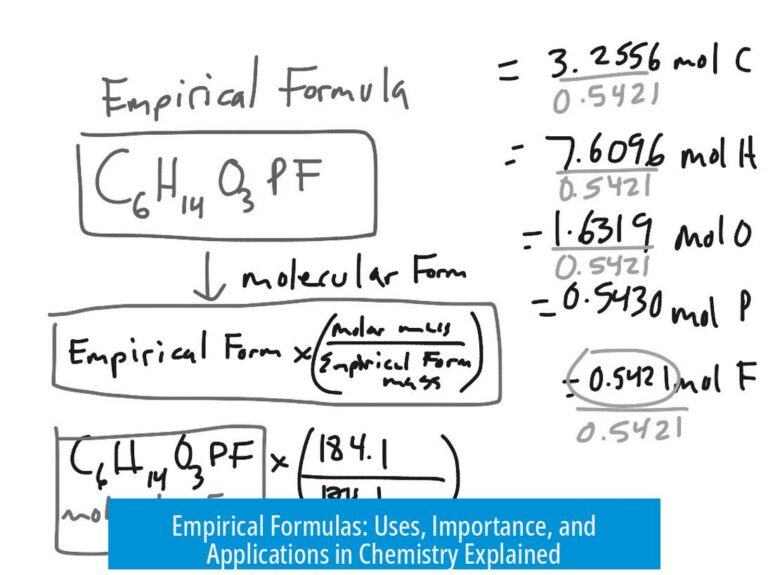
Why Two Hydrogen and Two Oxygen Form H2O: Understanding Chemical Reactions and Molecules
Why Does Two Hydrogen Plus Two Oxygen Form Water as H2O and Not Keep Oxygen as Two? The chemical formula for water is H2O because oxygen naturally exists as O2...