Neutrons are one of the most common particles in the universe and an integral part of any experiment involving nuclear fission. But have you ever wondered how big a neutron is? The size of neutrons has long been a mystery that cannot be determined through direct measurement—until now.
A team of researchers at the Institute of Theoretical Physics at Ruhr-Universität Bochum in Germany has taken a novel approach to calculating the size of neutrons, by combining their highly accurate calculations with recent measurements on light nuclei. Their results, which differ significantly from previous ones, have been published in the journal Physical Review Letters.
So, what are neutrons and how do they work? Neutrons and protons, commonly referred to as nucleons, form atomic nuclei and are made up of strongly interacting quarks and gluons. The size of a neutron is determined by its charge distribution, which is made up of positive and negative charge regions that, when taken together, result in zero total charge for the neutron.
To determine the size of the neutron, researchers have traditionally used scattering experiments with low-energy neutrons and heavy atoms, such as bismuth. However, the team at Ruhr-Universität Bochum have taken a different approach—calculating the deuteron radius with high accuracy from the lightest atomic nuclei. The deuteron is one of the simplest atomic nuclei and consists of one proton and one neutron.
In addition to providing insight into the structure of neutrons, knowledge of the size of neutrons is important for radiation protection strategies in nuclear reactors and other establishments. Neutron detection is also used for particle physics experiments and to monitor variations in cosmic ray flux.
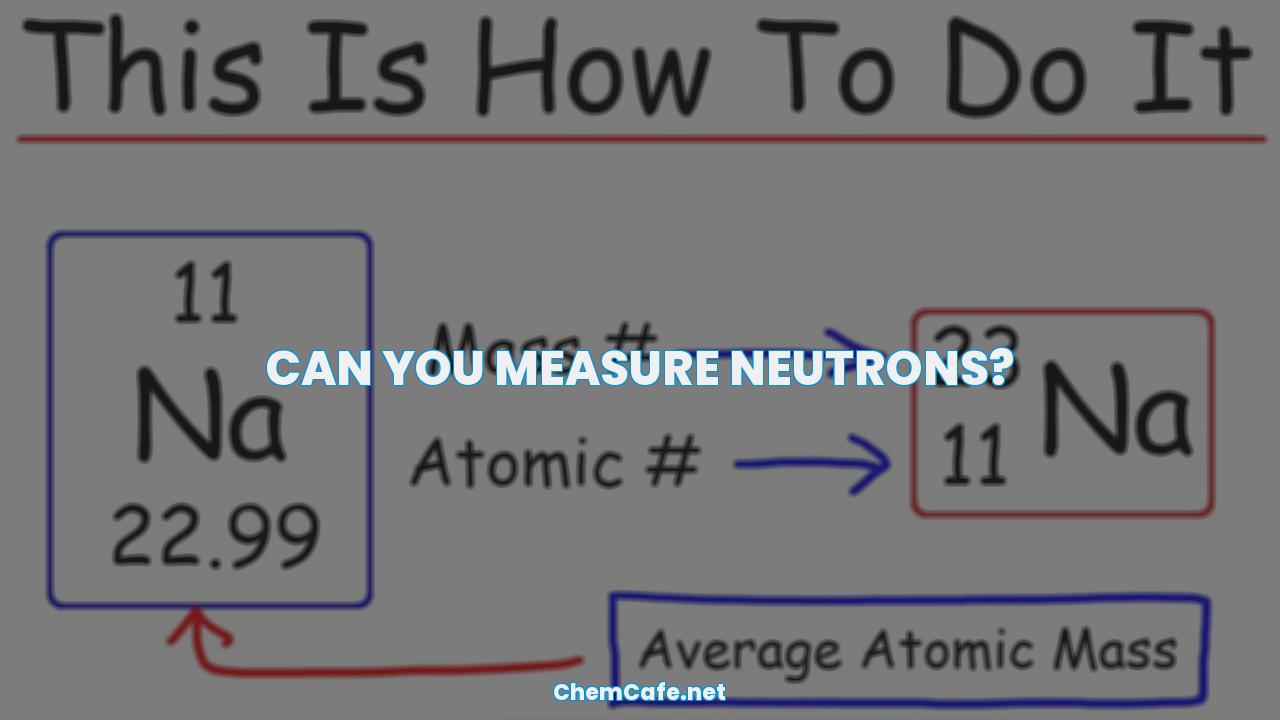
So, can you measure neutrons? Thanks to the work of the team at Ruhr-Universität Bochum, the answer is now a resounding yes.
Can you measure neutrons?
Neutrons are an integral part of our universe, forming atomic nuclei and playing a significant role in nuclear fission and radiation protection. But, one of the fundamental properties of neutrons is also one of the most difficult to measure – their size. In this blog, we’ll explore the current methods of measuring neutron size, as well as some of the challenges involved.
What is the size of a neutron?
The size of a neutron cannot be measured directly, but is instead determined from experiments involving other particles. Generally, this has been done in an indirect way, using measurements of heavier atoms. However, a team at the Institute of Theoretical Physics at Ruhr-Universität Bochum (RUB) have taken a different approach. By combining their accurate calculations with recent measurements of light nuclei, the researchers have developed a more direct methodology.
The results, which differ significantly from previous ones, were published in Physical Review Letters on 25th February 2020. As the team explains, neutrons and protons (also known as nucleons) are composed of strongly interacting quarks and gluons, and have a complex internal structure. The size of a neutron is determined by its charge distribution – the positive and negative charge regions which, when taken together, result in a neutron with zero total charge.
How is the size of a neutron measured?
The size of neutrons has traditionally been determined from scattering experiments with low-energy neutrons and electron shells of heavy atoms, such as bismuth. In this experiment, a neutron beam is directed at a target of heavy isotopes with many electrons, and the number of neutrons that pass through is measured.
The RUB team has now taken a different approach, by calculating the size of neutrons from the lightest atomic nuclei. The simplest atomic nuclei, the deuteron, consists of one proton and one neutron. As the two nucleons are relatively far apart, the deuteron is larger than its two constituents.
By combining their calculations with recent measurements of light nuclei, the team was able to determine the neutron charge radius from the deuteron. This resulted in a much more direct method of calculation, and yielded results that differ significantly from earlier ones.
Applications of neutron detection
Neutron detection is used for a variety of purposes, each of which has different requirements for the detection system. In nuclear power and research reactors, neutron flux is an important measure of power, and boiling water reactors may have dozens of neutron detectors – one per fuel assembly.
In particle physics, neutron detection has been proposed as a method of enhancing neutrino detectors. In radiation safety, neutron radiation is a hazard in nuclear reactors and neutron detectors must take into account the way damage caused by neutrons varies with energy.
Finally, neutron detectors are employed to monitor variations in cosmic ray flux, as secondary neutrons are one component of particle showers produced in Earth’s atmosphere by cosmic rays.
Challenges in neutron detection
Neutron detection is not an easy science. Detector hardware, such as the type of neutron detector used (often a scintillation detector) and the electronics used in the detection setup, must be taken into account. The hardware also defines key experimental parameters, such as source-detector distance, solid angle and detector shielding.
Detection software consists of analysis tools to measure the number and energies of neutrons striking the detector. Experiments making use of this science are typically scattering experiments whose scattered particles of interest are neutrons.
Perhaps the most noteworthy of these experiments is the European Muon Collaboration’s trademark experiment, first performed at CERN and now known as the ‘EMC effect’. This experiment is still performed today with more sophisticated equipment to obtain more definite results.
Conclusion
Measuring the size of neutrons is a complex process. While traditionally, measurements have been made in a very indirect way using heavy atoms, the team at RUB have taken a different approach, combining their calculations with recent measurements of light nuclei.
The results of this study, which differ significantly from earlier ones, provide us with a much more direct method of calculating the size of a neutron. Neutron detection is also used in nuclear reactors, particle physics, radiation safety and cosmic ray detection, with varying requirements for the detection system. However, effective neutron detection requires both hardware and software, and experiments involving neutrons are complex and require sophisticated equipment.
Despite these challenges, neutron detection remains an important science. The size, charge distribution and structure of neutrons are fundamental properties of our universe, and understanding them furthers our knowledge of the world we live in.
What do you mean by neutrons?
Neutrons are subatomic particles found in the nucleus of all atoms, except hydrogen. They have a mass similar to protons, but no electrical charge, and are symbolized as ‘n’ or ‘n0’. Neutrons were first discovered in 1932, and experiments conducted at the Stanford Linear Accelerator Center revealed that they are composed of smaller particles called ‘quarks’. Each neutron is made up of one ‘up’ quark and two ‘down’ quarks.
What Is the Purpose of a Neutron?
Neutrons serve several important purposes in the universe. They are found in the nucleus of atoms, along with protons, and the number of neutrons determines an atom’s isotope. Neutrons play an important role in nuclear reactions and nuclear power plants, and they can also be used to create medical isotopes for medical imaging.
What Are the Properties of Neutrons?
Neutrons have a mass of 1.67492729 x 10-27 kg and a spin of 1/2, which makes them a type of fermion. Even though a neutron has no electrical charge, it does contain charged components that cancel each other out. Additionally, neutrons can be ejected from the nucleus, though they do not last long before reacting with other atoms. On average, a free neutron will survive for about 15 minutes.
What Are the Uses of Neutrons?
Neutrons are used in a variety of ways. They are an important part of nuclear reactions, and they can be used to create medical isotopes for medical imaging. Additionally, they are used in neutron scattering experiments to study the properties of condensed matter and in neutron radiography to create images of objects that are otherwise difficult to see. Neutrons are also used in neutron spallation to create high-energy particles and in neutron activation analysis to detect trace elements in a sample.
How Are Neutrons Produced?
Neutrons are produced in a variety of ways. They can be produced naturally, through cosmic radiation from outer space, or artificially through particle accelerators, nuclear reactors, and nuclear weapons. Neutrons can also be produced through a process known as neutron activation, which involves bombarding a nucleus with neutrons to create a new nucleus with a different number of neutrons.
Conclusion
Neutrons are subatomic particles found in the nucleus of all atoms, except hydrogen. They have a mass similar to protons, but no electrical charge, and are symbolized as ‘n’ or ‘n0’. Neutrons are important in nuclear reactions and can be used to create medical isotopes for medical imaging. They are used in neutron scattering experiments to study the properties of condensed matter, in neutron radiography to create images of objects, and in neutron activation analysis to detect trace elements in a sample. Neutrons can be produced naturally, through cosmic radiation from outer space, or artificially through particle accelerators, nuclear reactors, and nuclear weapons.
Can neutrons make electricity?
Electricity is a vital part of our lives, and most of us take it for granted. We rely on it to power our homes, businesses, and even our cities. But how exactly is electricity made? It turns out that neutrons play a crucial role in the process.
What are neutrons and how do they work?
Neutrons are subatomic particles that are found in the nucleus of an atom. They are electrically neutral and have a mass of about 1.0087 amu. Neutrons interact with other particles in the nucleus through the strong nuclear force, which binds the nucleus together.
In nuclear fission, neutrons are used to split atoms and release energy in the form of heat. When atoms of uranium fuel are hit by neutrons, they fission (split) and release heat and more neutrons. Under controlled conditions, these other neutrons can strike more uranium atoms, splitting more atoms, and so on. Thereby, continuous fission can take place, forming a chain reaction releasing heat.
How is electricity made from neutrons?
The heat released by nuclear fission is used to turn water into steam, which is then used to spin a turbine connected to a generator. This is called a nuclear reactor. The generator produces electricity, which is then sent to a power station where it is distributed to the public.
In 2015, Nuclear power is used to generate 19.47 percent of all the country’s electricity. As of 2013, hydropower accounts for 6.8 percent of U.S. electricity generation. Its a process in which flowing water is used to spin a turbine connected to a generator. There are mainly two basic types of hydroelectric systems that produce electricity.
In the first system, flowing water accumulates in reservoirs created by the use of dams. The water falls through a pipe called a penstock and applies pressure against the turbine blades to drive the generator to produce electricity. In the second system, called run-of-river, the force of the river current (rather than falling water) applies pressure to the turbine blades to produce electricity.
Other Generating Sources
Geothermal power comes from heat energy buried beneath the surface of the earth. It is used to generate electricity by using steam produced from hot water and rocks beneath the earth’s surface to spin turbines. The steam is then used to spin a turbine connected to a generator, producing electricity.
How do neutrons behave in an electric field?
Because neutrons do not have an electric charge, they freely penetrate through the electron shells of atoms and are not repelled by the Coulomb field of the nucleus. When a neutron passes through the electric field, it is equivalent to the electric field moving towards a stationary neutron; only the perspective, or frame of reference, is different. And when the source of an electric field moves, it generates a magnetic field.
Atoms of all elements—except for most atoms of hydrogen—have neutrons in their nucleus. Unlike protons and electrons, which are electrically charged, neutrons have no charge—they are electrically neutral. Neutron does not get deflected in the presence of electric field. The neutron (with zero charge) experiences no electric force and, therefore, no acceleration.
Do neutrons play a role in electric charge?
Neutrons do not carry an electrical charge so adding or removing them from the nucleus does not change the electrical charge of the nucleus. It does, however, change the mass of the nucleus. Adding or removing neutrons from the nucleus are how isotopes are created.
Unlike the proton which has a positive charge, the neutron has no electrical charge and does not attract an electron to the atom. Although neutrons are not necessary for attracting electrons, they are required to space protons in the atomic nucleus.
Since neutrons are neither attracted to nor repelled from objects, they don’t really interact with protons or electrons (beyond being bound into the nucleus with the protons). Even though electrons, protons, and neutrons are all types of subatomic particles, they are not all the same size.
Complete answer: The protons, cathode rays and alpha particles are charged particles, so the magnetic field deflects them. Neutrons do not carry an electrical charge so adding or removing them from the nucleus does not change the electrical charge of the nucleus. It does, however, change the mass of the nucleus. Adding or removing neutrons from the nucleus are how isotopes are created.
Miller, a UW physics professor, has found that the neutron has a negative charge both in its inner core and its outer edge, with a positive charge sandwiched in between to make the particle electrically neutral. Protons are positively charged and are thus deflected on a curving path towards the negative plate. Electrons are negatively charged and are deflected on a curving path towards the positive plate. Neutrons have no charge, and continue on in a straight line.
We find that a large number of neutron transfer reactions indeed occur and impact crust models. In particular, we identify a new type of reaction cycle that brings a pair of nuclei across the nuclear chart into equilibrium via alternating neutron capture and neutron release, interspersed with a neutron transfer.
Neutrons are uncharged particles, and therefore they do not participate in the electromagnetic interaction and do not produce ionization of the atoms. Neutrons do not attract or repel each other, so they can’t collapse.
In conclusion, neutrons are an essential part of the process of generating electricity. They are used in nuclear fission to release energy, which is then used to generate electricity in nuclear reactors and hydroelectric power plants. Neutrons, being uncharged particles, do not participate in the electromagnetic interaction and do not produce ionization of the atoms. They are also used to create isotopes, which is a process of adding or removing neutrons from the nucleus. Neutrons also play an important role in stabilizing the nucleus of an atom, as they provide spacing between the protons.
How neutrons are in an atom?
Neutrons are one of the most important components of an atom. They are the largest of the particles that make up the atom, and they are found in the nucleus of the atom with protons. Neutrons have a neutral charge, and they are held together with the protons in the nucleus by a force called the strong nuclear force. The nucleus is a very dense area located in the center of the atom.
Neutrons are often overlooked when discussing atomic structure, but they are essential for understanding the behavior of an atom. In this article, we will explore what neutrons are, where they are located in an atom, and how they are studied.
What are Neutrons?
Neutrons are a type of baryon, which means they are made up of 3 quarks (1 up quark and 2 down quarks). Quarks are the building blocks of all matter, and they are responsible for forming all the components of an atom. Neutrons have a rest mass equal to 1.67492749804 × 10-27 kg, which is slightly greater than that of the proton but 1,838.68 times greater than that of the electron.
The neutron has a magnetic dipole moment, which means it behaves like a tiny magnet in ways that suggest it is an entity of moving electric charges. Neutrons also have no electric charge, and they are the only particles that form the nucleus of an atom along with protons.
Where are Neutrons Located in an Atom?
Neutrons are located in the nucleus of the atom with protons. The nucleus is a very dense area located in the center of the atom. It is packed tightly with protons and neutrons, and the protons and neutrons are held together in the nucleus by a force called the strong nuclear force.
How are Neutrons Studied?
Neutrons are studied using a variety of techniques. Scientists produce neutrons at research reactors and particle accelerators and then project them onto samples of materials. When the neutrons interact directly with atoms in the sample, they bounce away at different angles, like cue balls colliding in a game of pool. This technique is called neutron scattering.
Scientists can then use special high-speed detectors to capture the scattered neutrons and measure their energy, speed, and direction. This information helps researchers calculate the materials’ properties, such as the shape and sizes of crystals and molecules.
Neutrons are also used in medical research and imaging. Neutrons can penetrate deeper into tissue than X-rays, making them useful for imaging deeper structures. Neutron scattering and imaging can also be used to study the structure of proteins and other complex molecules.
Conclusion
Neutrons are an essential component of an atom, and they are located in the nucleus with protons. They have a neutral charge and a rest mass slightly greater than the proton but 1,838.68 times greater than that of the electron. Neutrons are studied using a variety of techniques, such as neutron scattering and imaging. These techniques help scientists understand the structure of materials, proteins, and other complex molecules.
Is a neutrons mass 1?
Neutrons are one of the three subatomic particles found in atoms, together with protons and electrons. These particles have different masses and charges, and consequently, many properties. The mass of a neutron is one of the most important of these properties, as it affects the stability and properties of an atom.
Mass of a Proton and Neutron
The mass of a proton is 1.6726231 x 10⁻²⁷ kg whereas the mass of the neutron, mn = 1.6726231 x 10⁻²⁷ kg. This means that the mass of a neutron is almost the same as that of a proton. However, the mass of a neutron is slightly higher than that of a proton due to the binding energy of the neutron-proton pair, which is known as the mass defect.
Mass of Neutron in Grams
We know the mass of neutron in kg is 1.6726231 x 10⁻²⁷ Kg. We also know that 1 kg = 10³ g. So, mass of neutron in grams = 1.6726231 x 10⁻²⁷ x 10³.
mn= 1.6726231 x 10⁻²⁴ g
Mass of Neutron in Amu
The rest mass of the neutron that we have calculated above is in the unit of kg. In amu or atomic mass unit, the mass of neutron is calculated as, Since 1 kg = 6.0229552894949E+26 amu. So, 1.6749286 x 10⁻²⁷kg = 1.6749286 x 10⁻²⁷ x 6.0229552894949E + 26. mn (amu) = 1.008664904(14) amu
Rest Mass of Neutron
The concept of rest mass is very simple. We generally think of mass as being a constant quantity for an object. However, the theory of relativity tells us that energy and mass are interchangeable. It means that the mass of a body increases with the increase in its velocity relative to the observer. Energy gets affected by increasing an object’s mass. So, the minimum mass of an object is when it is stationary.
Charge and Mass of a Neutron
A neutron has no electric charge attached to it. As a result, neutrons are subatomic particles with neutral charges. A neutron has a mass equivalent to 1.008 atomic mass units. The estimated mass of a neutron can be 1.674 * 10-27 kg when expressed in kilograms. The analytical method of mass spectrometry cannot easily measure the mass of neutrons since they have no electric field. Deuterium, a hydrogen isotope, has one proton, one neutron, and one electron as part of its atomic structure. The mass of a deuterium nucleus can be calculated by deducting the mass of a proton from that of a deuterium nucleus. Because the electron’s mass is extremely small compared to the masses of the neutron and proton, the mass of a neutron can be estimated by deducting the mass of a proton from the mass of a deuterium atom.
Mass Neutron
What is the mass of a neutron? Well, mass spectroscopy cannot directly determine the mass neutron. But this can be determined using deceptive techniques. Mass Neutron (mN) = 1.674927471(21) * 10-27 kg.
What is the Mass of a Neutron?
Neutron Mass – Explanation and Important FAQs
Neutrons are subatomic particles with no electrical charge. They are found in the nucleus (center) of atoms, together with protons.The word neutron comes from the Greek word νέος ( neutrós ), meaning “neutral” since they have no charge and hence interact weakly with other particles.Neutrons have a mass of 940.6 MeV/c² or approximately 1.6749 × 10−27 kg on the atomic mass unit (u) scale. By contrast, protons have a rest mass of 0.93887 u.
Formation
The neutrons in the cosmos are formed by fusion in the core of stars.When a very high-energy cosmic ray proton collides with an atomic nucleus, it can produce a neutron and a hydrogen nucleus (H) in a reaction that may be written as follows:proton + atomic nucleus → neutron + H
There is another reaction, the opposite one, where a high-energy neutron collides with an atomic nucleus, producing a proton, an electron (e) and an anti-neutrino (ν):neutron + atomic nucleus → proton + e + anti-neutrinoThese newly created particles are on their own, typically moving at great speed.
Mass of Neutron | Properties of Neutrons | About Neutron
Mass of Neutron Chemistry Formulas Relative masses of protons, neutrons and electrons Sub atomic particles are extremely small and almost mass less. Still if we compare the mass of protons, neutron and electron it is observed that mass of Neutrons and Protons are almost equal whereas mass of electron are very less as compared to mass of proton and Neutrons. Therefore when we calculate amu the mass of electron is negated.
Relative Mass of Proton Neutron and electron are
In this relative mass calculation we have assume that a neutron has a mass of 1, then the relative masses of proton and electron are. Neutron = 1 Proton = 0.99862349 Electron = 0.00054386734
Mass of Neutrons In Kg
Neutron = 1.6749286*10-27 kg Proton = 1.6726231*10-27kg Electron = 9.1093897*10-31 kg
Mass of Neutron in MeV
Neutron = 939.56563 MeV Proton = 938.27231 MeV Electron = 0.51099906 MeV
Fundamental Properties of Proton neutron and Electron
1. Electron: Electron is a universal constituent of matter. Discovery: It was discovered by J.J. Thomson in 1886, through the study of cathode rays (Julius Plucker) and the name electron was proposed by Stoney.
To sum up, the mass of a neutron is 1.008664904(14) amu or 1.6749286*10-27 kg. This mass is slightly higher than the mass of a proton due to the binding energy of the neutron-proton pair. Neutrons are subatomic particles with no electrical charge, and are found in the nucleus (center) of atoms, together with protons. The neutrons in the cosmos are formed by fusion in the core of stars, and can be produced in a reaction where a very high-energy cosmic ray proton collides with an atomic nucleus.
Do neutrons have a charge?
The answer to the question “Do neutrons have a charge?” is a bit more complicated than a simple yes or no. Neutrons are a type of subatomic particle that form the nucleus of an atom, along with protons. Electrons, the third type of subatomic particle, orbit the nucleus. So, what is the charge of a neutron?
Neutrons Have a Neutral Charge
The charge of a neutron is neutral, meaning it is neither positive like the protons, nor negative like the electrons. Neutrons have no electric charge, so they don’t interact with electric fields. This means they are affected only by the strong nuclear force, which binds the protons and neutrons together in the nucleus of the atom.
The Structure of a Neutron
So, what is the structure of a neutron? A neutron is made up of three quarks, two of which have a negative charge of 1/3 each, and one with a positive charge of 2/3. When these charges are added up, they cancel each other out, giving the neutron a net charge of zero.
Neutrons and Electromagnetism
Despite having a neutral charge, neutrons do interact with electromagnetism. Experiments conducted in particle accelerators suggest that the neutron is more like an onion when it comes to electromagnetism: with a negatively charged exterior and interior and a positively charged middle sandwiched between them. Even though the sum of these charges cancel each other out, the findings change our understanding of how neutrons interact with the other particles in the atom.
The Significance of Neutrons’ Charge
The charge of a neutron is significant because it affects how it interacts with other particles. For example, when a particle like an electron interacts with a neutron, where it hits will affect how it behaves and where it goes. This means that when we study the structure of the atom, we must consider the charge of the neutron as well.
Conclusion
To answer the question “Do neutrons have a charge?”, we can say that neutrons have a neutral charge. This is because the charges of the three quarks that make up the neutron cancel each other out, giving the neutron a net charge of zero. Despite this, neutrons do interact with electromagnetism, and the charge of a neutron affects how it interacts with other particles. Understanding the charge of a neutron is essential to understanding the structure of the atom.
What is the charge of the neutron?
The neutron is one of the three subatomic particles that form atoms and is located in the nucleus of the atom with the protons. Neutrons have a neutral charge and are neither positive, like the protons, nor negative, like the electrons. The charge on a proton is +e, while the charge on an electron is -e and the charge on a neutron is 0.
Understanding the Charge of a Neutron
The charge of a neutron is 0. This means that the neutron has no electrical charge, meaning that it is neither attracted nor repelled by an electrical field. This is important for the stability of an atom, as the positive charge of the protons is balanced by the negative charge of the electrons, and the neutral charge of the neutrons.
The neutron is composed of two down quarks, each with 1/3 elementary charge, and one up quark, with 2/3 elementary charge. This combination of quarks gives the neutron a neutral charge. The nucleus of an atom is bound together by the residual effect of the strong force, which is a fundamental interaction that governs the behaviour of the quarks that make up the individual protons and neutrons.
Neutron Discovery
The neutron was discovered in 1932 by the English physicist James Chadwick. Chadwick was awarded the Nobel Prize in Physics in 1935 for this discovery. He was able to prove the existence of neutrons by bombarding beryllium with alpha particles. This experiment showed that a new particle was being created, which was later identified as the neutron.
Neutrons are not only found inside the nucleus of an atom, but they can also exist as free particles outside of the nucleus. This is known as a free neutron. Free neutrons can be created in nuclear reactors, particle accelerators, and in nuclear fusion reactions. These free neutrons can then be used to create a variety of different elements.
The Role of Neutrons in Nuclear Reactions
Neutrons play an important role in nuclear reactions. For example, in a nuclear fission reaction, neutrons are used to split the nucleus of an atom into two smaller nuclei. This process releases a large amount of energy and is used in nuclear power plants to generate electricity.
In a nuclear fusion reaction, two smaller nuclei are combined to form a larger nucleus. This process also releases a large amount of energy and is used in hydrogen bombs. The neutrons released in this reaction can also be used to create new elements.
Conclusion
The neutron is an essential part of an atom and has a neutral charge. It is made up of two down quarks and one up quark and is bound together in the nucleus of the atom by the strong force. Neutrons play an important role in nuclear reactions, such as nuclear fission and nuclear fusion.
Can you break a neutron?
Neutrons are among the most important particles in the observable universe, forming the nucleus of atoms. These neutrons are held together by a strong nuclear force and are incredibly difficult to break apart, but is it possible to break a neutron?
To answer this question, we first need to understand the structure of a neutron and how it interacts with other particles. A neutron is composed of three quarks, two up quarks and one down quark. These quarks are held together by the strong nuclear force and are what give the neutron its mass and stability. When a neutron is outside of a nucleus, it is unstable and will eventually break down into a proton, an electron, and an antimatter neutrino. This process is known as beta decay and is the most common way for a neutron to break down.
Is it possible to break a neutron?
Yes, it is possible to break a neutron, but it is not an easy task. Generally, neutrons are broken down in a process known as beta decay. In this process, a neutron decays into a proton, an electron and an antimatter neutrino. However, there are more exotic ways to break down a neutron. One such process is through a beam experiment, where a beam of neutrons is shot into a magnetic trap that catches positively charged protons. This process is used in particle accelerators to study the structure of the neutron.
Can a neutron star be broken?
Neutron stars are incredibly dense objects composed mostly of neutrons. These stars are so dense that the pressure of the neutrons is enough to counter the gravitational pull of the star, allowing it to remain stable. However, there is a limit to the mass of a neutron star and if the core is more massive than that limit, the neutron pressure will be overwhelmed by gravity and the star will collapse into a black hole. So while it is possible to break a neutron star, it requires a great deal of energy to do so.
Can you split a proton or neutron?
Protons and neutrons are made up of quarks and gluons, which are the building blocks of atomic nuclei. Currently, scientists believe that quarks and gluons are indivisible and cannot be broken down into smaller components. This means that it is not possible to split a proton or neutron.
What if you touch a neutron?
Neutrons are found in the nucleus of an atom and, under normal conditions, protons and neutrons will stick together. However, during radioactive decay, they may be knocked out of the nucleus. If you were to touch a neutron, it is unlikely that anything would happen as neutrons have no electric charge and will not interact with matter in the same way that an electron or proton would.
Is it impossible to breakup atoms?
Contrary to the literal meaning of the word “atom”, which means indivisible, quantum mechanics allows for atoms to be divided and reunited. Researchers have recently demonstrated that it is possible to split a single atom into its two halves, pull them apart, and then put them back together again.
Can a black hole rip a neutron star?
When a neutron star meets a black hole that is much more massive, such as the recently observed events, the two will circle each other in a spiral. Eventually, the black hole will swallow the neutron star. The gravitational waves that were observed back in 2015 indicate that such an event is possible, though it is still not clear how much of the neutron star would be destroyed and how much would remain.
How heavy is a teaspoon of a black hole?
A teaspoon of a black hole would be incredibly heavy, as black holes are incredibly dense objects. In fact, a teaspoon of a black hole would weigh more than five billion tons, or the equivalent of a million elephants.
What is the neutron bomb?
The neutron bomb is a type of weapon that is designed to produce a large amount of radiation while limiting the destruction caused by the blast. This is done by increasing the amount of neutrons released during the explosion, which causes a greater amount of radiation damage, but less overall destruction. The neutron bomb was tested successfully in 1962, but it has yet to be used in an actual conflict.
Can neutrons hurt you?
Yes, neutrons can hurt you. Neutrons are highly energetic particles and can cause cells to change their functionality or stop replicating altogether. This can cause damage to the body over time and neutrons are particularly damaging to soft tissues such as the cornea of the eye.
Is there anything smaller than a neutron?
Yes, there are things that are smaller than a neutron. Quarks are the smallest particles in the universe and they are much smaller than the protons and neutrons in which they are found. Quarks are indivisible and operate at much higher energy levels than neutrons.
Is a neutron bomb a nuke?
Yes, the neutron bomb is a type of nuke. It is a small hydrogen bomb that is designed to release a large amount of radiation while limiting the destruction caused by the blast. It is also known as an enhanced-radiation weapon (ERW).
What is the hottest thing in the universe?
The hottest thing in the universe is a supernova. These are incredibly powerful explosions that occur when a star dies. During a supernova, the temperatures at the core can reach up to 6000 times the temperature of the sun’s core.
What’s the smallest thing humans can see?
The smallest thing humans can see is the wavelength of visible light, which is around 400 nanometers. This means that humans can see objects that are as small as 400 nanometers in size.
Is it possible to break apart a neutron star?
Yes, it is theoretically possible to break apart a neutron star through a process known as recycling. This process involves shooting a beam of neutrons into a magnetic trap that catches the protons. However, this process is incredibly difficult and would take a great deal of energy to accomplish.




Leave a Comment