In the world of chemistry, the periodic table is an essential tool for understanding the fundamental structure of matter. It’s a way to put order and meaning to the seemingly chaotic array of elements that make up our universe. But what lies beyond the 118 elements we’re familiar with? Is it possible to create elements with more protons than those found in naturally occurring elements?
Element 120, also known as unbinilium, is a hypothetical element that has been theorized to exist but has never been observed in nature. If it were able to be synthesized, it would be the first element beyond the traditional 118 elements of the periodic table. Scientists have attempted to synthesize element 120 in the lab by adding together two different elements: titanium-50 with 22 protons and californium-249 with 98 protons. However, the probability of survival for such a process is extremely weak.
The same applies for element 121, also known as unbiunium, which has been theorized to exist but has yet to be observed. Even if it were possible to synthesize element 121, the question remains whether it would be stable enough to exist in nature. Elements above atomic number 92, uranium, are generally considered to be radioactive and extremely unstable.
But what’s more, the question of element 120 and beyond begs the exploration of whether there are any elements beyond atomic number 118 that can have 10 stable isotopes. If so, these elements could have a much greater range of applications and uses in chemistry.
The truth is, element 120 and beyond are still largely a mystery. Theoretical models have predicted elements beyond 118, but these predictions have yet to be tested. Until we can observe the existence of element 120, the answers to these questions may remain a mystery.
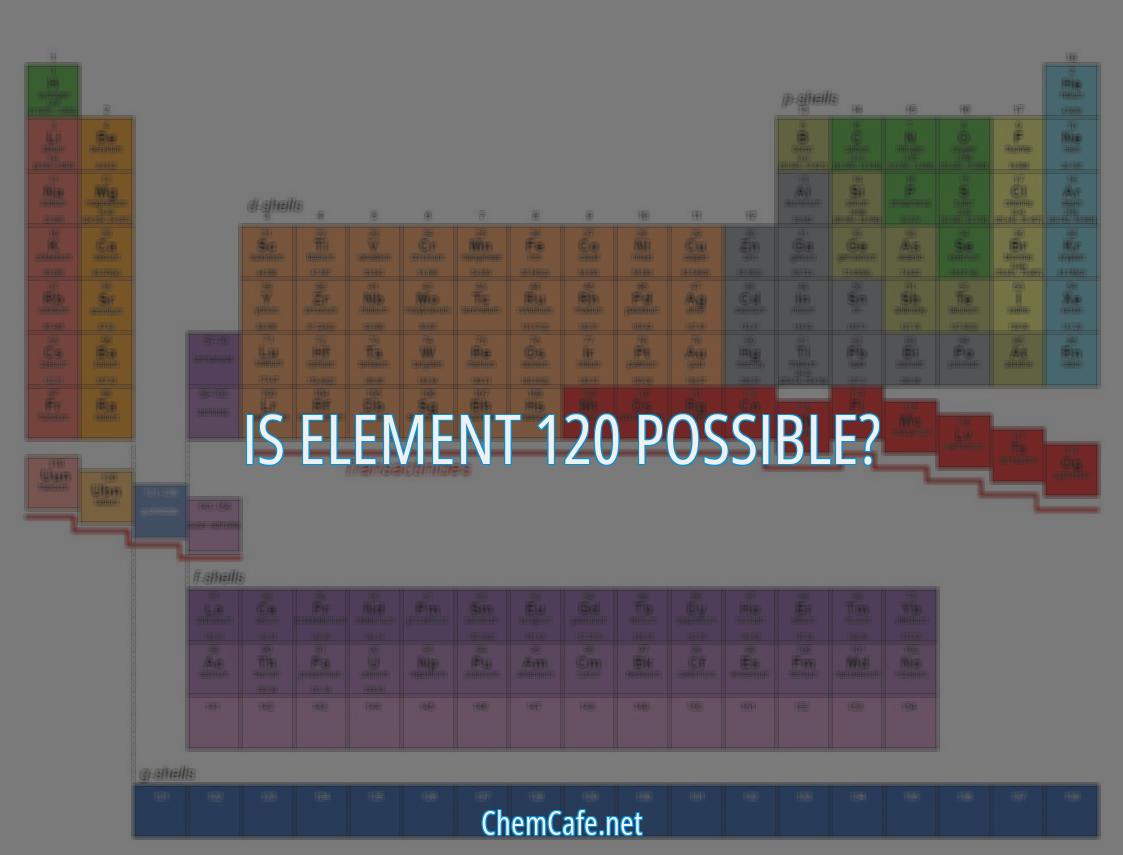
Is element 120 possible?
The periodic table of elements is a chart that organizes all the known chemical elements in order of atomic number. The current table consists of 118 elements, with elements 119 and 120 still not discovered or accepted as part of the periodic table. This raises the question: is element 120 possible?
What is element 120?
Element 120 is a hypothetical element that has yet to be found. It would be the highest atomic number element on the periodic table, with a total of 120 protons. Its chemical symbol is Ubu, and it would be part of the 7th period of the periodic table.
Scientists have theorized that element 120 could exist and have attempted to create it in labs. However, the probability of its survival is extremely weak due to the instability of the nucleus. This means that it is difficult to create and sustain the element, making it difficult to research.
What is needed for element 120 to exist?
In order for element 120 to exist, two elements must be combined. Specifically, a titanium-50 beam (Z=22) and a californium-249 target (Z=98). When these two elements are combined, they create 120 protons. But why are these two elements chosen?
The reason is that these two elements have a high probability of fusing together. The titanium-50 beam has a high probability of penetrating the californium-249 target, and the californium-249 target has a high probability of fusing with the titanium-50 beam. This makes them the best combination for creating element 120.
Is element 126 possible?
Element 126, also known as unbiunium, is a hypothetical chemical element with an atomic number of 126. As of 2020, no elements with an atomic number greater than 118 have been successfully incorporated into the periodic table. However, extended periodic table theories have predicted the existence of elements beyond 118.
According to these theories, element 126 is a chemical element with an atomic number of 140. It is still unknown if element 126 is possible, as research has yet to be conducted. It is speculated that element 126 could be created in a lab using a combination of two elements with a high probability of fusing together.
Conclusion
Element 120 is a hypothetical element that has yet to be discovered and accepted as part of the periodic table. In order for element 120 to exist, two elements must be combined: a titanium-50 beam (Z=22) and a californium-249 target (Z=98). Though researchers have attempted to create element 120, the probability of its survival is extremely weak. Element 126 is also a hypothetical element with an atomic number of 126, and its possibility is still unknown.
Can an element have 119 protons?
Atoms are the smallest components of matter, and their structure is determined by the atomic number, which is equal to the number of protons in the nucleus. We currently know that atoms with atomic numbers up to 118 are possible, but can atoms exist with an atomic number of 119?
Theoretical Arguments for an Element with 119 Protons
Theoretically, there could be an element with 119 protons, just as there could be elements with higher atomic numbers. In fact, researchers have already seen increasing stability of known superheavy elements when in isotopes with neutron numbers closer to the magic 184. This suggests that it’s possible to create an element with 119 protons, though it may be difficult to do so.
The Periodic Table and the Element 119
The periodic table is often used to predict the properties of elements and can provide insight into why an element with 119 protons might not be possible. For example, technetium (atomic number 43) and promethium (atomic number 61) have no stable forms while the elements surrounding it in the periodic table do have stable forms. This suggests there may be a gap in the periodic table that would prevent an element with 119 protons from existing.
Heuristic Arguments Against an Element with 119 Protons
In addition to theoretical arguments, there are also heuristic arguments against an element with 119 protons. Heuristic arguments are based on experience and observation, and suggest that an element with 119 protons may not be possible due to the increasing instability of elements as the atomic number increases.
The Difficulty of Creating an Element with 119 Protons
Given the theoretical and heuristic arguments against an element with 119 protons, it is unlikely that such an element can exist. Even if it were theoretically possible, creating an element with 119 protons would be extremely difficult. This is because it would require two nuclei that could give us a superheavy element that also has a large number of neutrons.
Conclusion
Based on the arguments discussed in this article, it is unlikely that an element with 119 protons is possible. Even if such an element were theoretically possible, it would be extremely difficult to create it due to the difficulty in finding two nuclei that could give us a superheavy element with a large number of neutrons. However, more research is needed to definitively answer the question of whether an element with 119 protons is possible.
Why is there no element 119?
The periodic table of elements is a cornerstone of modern chemistry, and it’s a great way to visually represent the different elements and their properties. But have you ever wondered why there is no element 119?
Unbibium, also known as eka-thorium or element 122, is the currently hypothetical chemical element with the placeholder symbol of Ubb and atomic number 122. Scientists have long debated the potential existence of this element, and why it has yet to be discovered.
Can We Run Out of Elements?
It’s unlikely that we’ll ever deplete the universe—or even our own little planet—of any particular element in its entirety. But we don’t have to literally go dry for serious supply issues to arise.
Element 119 is a particularly interesting case, because it has been attempted to be synthesized in a laboratory. In 1985, an attempt was made at the superHILAC accelerator at Berkeley, California to make element 119 from calcium-48 and less than a microgram of einsteinium. Unfortunately, the experiment failed.
Would Element 119 Be a Metal?
Element 119 is expected to be a typical alkali metal with a +1 oxidation state. Scientists have theorized that it would have similar properties to other alkali metals such as sodium, potassium, and rubidium, meaning it would be soft, silvery-white, and highly reactive.
Will There Be a 120th Element?
While there is currently no consensus on the placement of elements beyond atomic number 120 in the periodic table, scientists have theorized that a 120th element could exist. This element has been theorized to potentially fill a gap in what’s known as the “island of stability”, where certain elements are predicted to have longer-than-expected lifetimes.
What Would Element 119 Be?
So where will we place element 119 in the periodic table of elements? Based on both the Seaborg and Pyykkö extended periodic tables described above, element 119 will be the start of period 8 and it will be an alkali metal.
Where Does the Periodic Table End?
At this point, no one knows where the periodic table ends. Scientists have theorized that element 126 is the “super-heavy” element that marks the end of the periodic table, but this is still theoretical.
What is Earth’s Rarest Element?
Earth’s rarest element is astatine, which has an average concentration of only 0.25 parts per quadrillion in the environment. Astatine is a naturally occurring radioactive element that is found in trace amounts in uranium ore deposits, but it is not found in significant concentrations anywhere on Earth.
Element 119 is still a theoretical element, and its discovery is still up in the air. Scientists will continue to search for it, and other undiscovered elements, in order to better understand the universe around us. Until then, element 119 will remain a mystery.
Is element 119 possible?
The periodic table of elements is an essential tool for understanding the properties of different elements, and it is constantly being updated and revised as new elements are discovered. But what of element 119 – is it possible?
Element 119 is expected to be a typical alkali metal with a +1 oxidation state, and an attempt to make it from calcium-48 and less than a microgram of einsteinium was made in 1985 at the superHILAC accelerator at Berkeley, California. Unfortunately, it did not succeed, but that doesn’t mean that element 119 isn’t possible.
Where Will We Place Element 119 in the Periodic Table of Elements?
There are two main ways of extending the periodic table beyond element 118 – the Seaborg and Pyykkö extended periodic tables. According to both of these extended tables, element 119 will be the start of period 8 and it will be an alkali metal.
Will There Be a 120th Element?
While we haven’t yet succeeded in making element 119, there is still a chance that a 120th element could exist. There is currently no consensus on the placement of elements beyond atomic number 120 in the periodic table, as there is not enough data to accurately predict the properties of such elements.
What Would Element 119 Be?
If element 119 were to be successfully synthesized, it would likely be called unbihexium, also known as element 126 or eka-plutonium. This element has the atomic number 126 and a placeholder symbol of Ubh. Calculations have shown that 326Ubh would be the most stable isotope.
Similarly, element 123, also known as unbitrium or eka-protactinium, has the temporary symbol Ubt and has the atomic number 123. Calculations have shown that 326Ubt would be the most stable isotope.
What is Earth’s Rarest Element?
Earth’s rarest element is astatine, which is an extremely rare element that is found in very small amounts in nature. It has an atomic number of 85 and is a member of the halogen family. Astatine is a radioactive element and is highly toxic, so it is not used in many applications.
Conclusion
Element 119 is yet to be successfully synthesized, but scientists are continuing to try. If element 119 were to be created, it would likely be an alkali metal and have an atomic number of 119. Additionally, element 123 is also possible, and it would have an atomic number of 123. Finally, astatine is the rarest element found on Earth.
Why are elements above 92 unstable?
Radioactive isotopes are unstable atoms that emit ionizing radiation. Most elements that are found in nature are made up of stable isotopes, meaning they don’t decay over time. Tin is the element with the most stable isotopes, with ten different stable isotopes. But what about the elements that are above 92 on the Periodic Table? Why are they unstable?
Interesting Facts About Isotopes
When an element is radioactive, it means it only exists in an unstable form. All non-natural elements are radioactive isotopes. Additionally, heavier isotopes tend to react more slowly than lighter isotopes of the same element.
When it comes to the number of isotopes an element can have, the answer is variable. Hydrogen has the smallest number of isotopes with only three. The elements with the most isotopes are cesium and xenon, with 36 known isotopes.
Stable and Unstable Isotopes
Some isotopes are stable, while others are unstable. Unstable isotopes will decay over time, eventually turning into another isotope or element. Generally, the most stable form of an element is the most common in nature. However, all elements have an unstable form.
These unstable elements, called radionuclides, emit ionizing radiation and are radioactive. Some elements, like uranium, don’t have a stable form and are always radioactive. When a radioactive element decays, it transforms into a different atom, a decay product.
Why Are Elements Above 92 Unstable?
Elements above 92 on the periodic table are unstable because they have more protons in their nucleus than the elements below them. This means they have more positive charges in their nucleus than negative charges, making them more likely to decay.
Adding more protons to a nucleus affects the ability of the nucleus to hold together. This is because the protons in the nucleus repel each other due to their positive charges, making it harder for the nucleus to stay stable. This is why elements above 92 on the periodic table are less stable than those below them.
In conclusion, elements above 92 on the periodic table are unstable because they have more protons in their nucleus than the elements below them. This causes the protons to repel each other, making it harder for the nucleus to stay stable. As a result, these elements are more likely to decay over time.
Are there 10 stable isotopes?
Stable isotopes are atoms of the same element that have the same number of protons, but different numbers of neutrons. Most of the naturally occurring isotopes are considered to be stable, but there are a few tens of them that are radioactive with very long half-lives. In order for a nuclide to be present in the natural isotopic composition of a chemical element, its half-life must be comparable with or more than the age of the Earth (4.5 billion years). For example, the half-life of uranium-235 is 700 million years.
Types of Isotope Fractionation
When isotopes are fractionated, they can be separated into three different types: mass-dependent fractionation, mass-independent fractionation, and kinetic fractionation. Mass-dependent fractionation occurs when the lighter isotopes are preferentially fractionated from the heavier ones due to the physics of their mass. Mass-independent fractionation occurs when chemical processes are responsible for the fractionation of isotopes. Finally, kinetic fractionation occurs when chemical reactions have different rates for different isotopes.
List of Stable Isotopes
As of September 2007, there were 244 known stable isotopes. Xenon is the only element that has nine stable isotopes, while tin has the most stable isotopes with 10. No element has exactly eight stable isotopes. Eighty out of the first 82 elements in the periodic table have stable isotopes. By measuring and analyzing their distribution, many practical applications can be developed.
Practical Applications of Stable Isotopes
Stable isotopes can be used in many practical applications, such as hydrology, environmental studies, and agriculture. The International Atomic Energy Agency assists Member States in using isotope-based techniques in these areas. Stable isotopes can be measured by analyzing the amounts and proportions of isotopes in samples, such as water samples. This type of analysis can help researchers learn more about the environment, such as the source of water and the age of the water.
In conclusion, there are 10 stable isotopes of tin, which is more than any other element. There are also 244 known stable isotopes in total, with 80 of the first 82 elements in the periodic table having at least one stable isotope. Stable isotopes can be used in many practical applications, such as hydrology, environmental studies, and agriculture. The International Atomic Energy Agency assists Member States in using isotope-based techniques in these areas.



Leave a Comment