What Does Delocalized Pi Bond Mean?
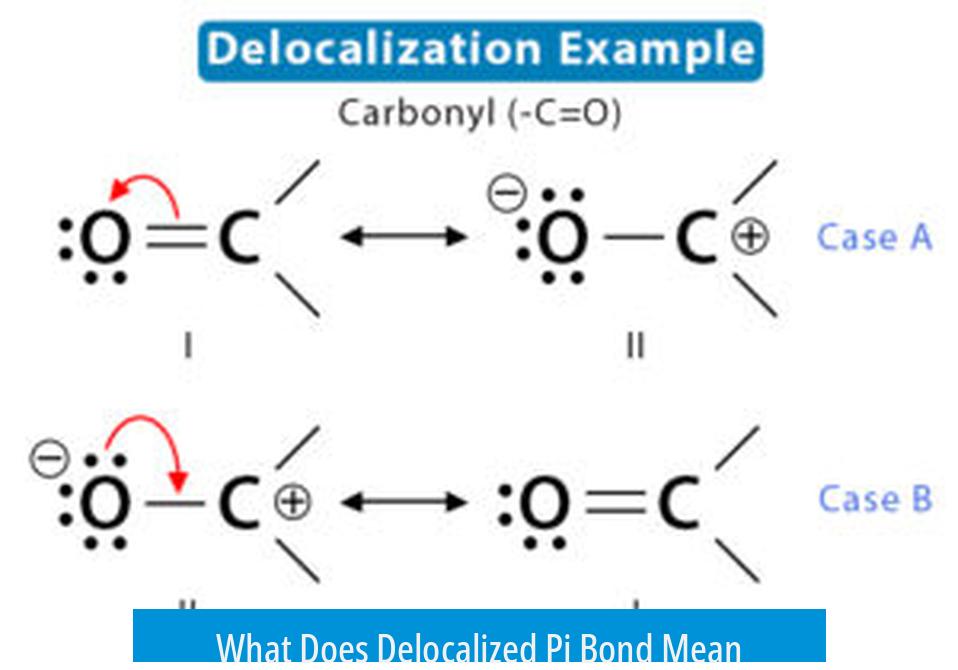
A delocalized pi bond refers to a set of pi electrons shared among multiple atoms rather than confined between two atoms. This phenomenon is typical in aromatic compounds like benzene, where pi electrons form a continuous, overlapping electron cloud around a ring structure, increasing molecular stability.
Understanding Delocalized Pi Bonds
Pi bonds (π bonds) arise from the sideways overlap of p orbitals, creating regions of electron density above and below the atomic nuclei. Normally, these pi electrons are localized between two carbon atoms forming a double bond. Delocalization means the pi electrons are no longer restricted to one bond; they extend over multiple atoms.
This behavior is most pronounced in aromatic compounds, such as benzene. Instead of having alternating single and double bonds fixed between pairs of carbon atoms, the pi electrons circulate freely above and below the carbon ring. This creates a uniform distribution of electron density, significantly different from localized bonds.
Mechanism of Pi Electron Delocalization
In a benzene ring, each carbon atom contributes one p orbital with a single electron. These orbitals overlap sideways, forming a continuous ring of overlapping pi orbitals.
- Each pi electron is not tied to any one C-C pair.
- The electrons become part of a collective electron cloud encompassing all six carbons.
- This creates an extensive conjugated system where electrons flow over the entire ring.
This overlap forces the electrons to be shared evenly, resulting in identical bond lengths among the carbon atoms, in contrast to typical alternating single and double bonds seen in isolated alkenes.
Resonance and Movement of Pi Bonds
Delocalized pi bonds can be explained by resonance theory. Classical structures show alternating double and single bonds, but the true structure results from a hybrid of these. The pi bonds effectively ‘move’ around through resonance.
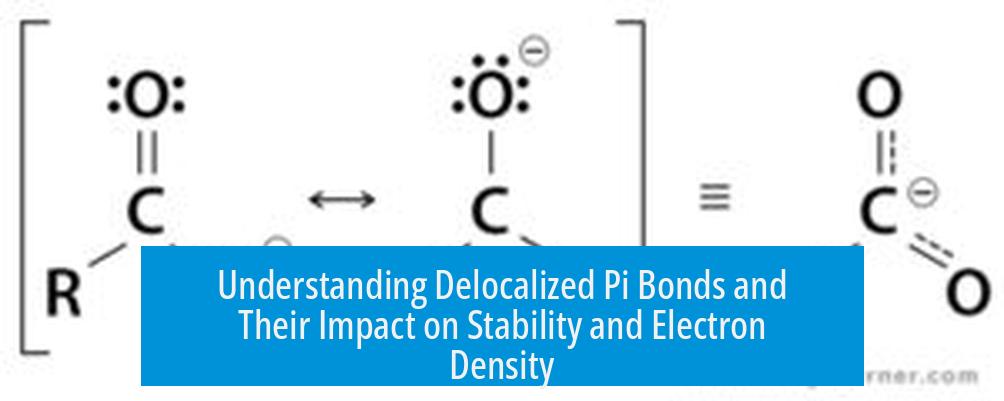
For example, in a carboxylate ion, resonance allows the negative charge and pi electrons to be distributed between two oxygen atoms, stabilizing the ion. This illustrates how delocalization spreads electron density rather than confining it.
Delocalized Pi Electrons Increase Stability
Delocalization adds stability by lowering the overall energy of the molecule. The electron cloud spread across several atoms reduces electron repulsion and decreases reactivity.
Benzene’s six-carbon ring is a classic example:
| Property | Localized Double Bonds | Delocalized Pi Bonds |
|---|---|---|
| Bond Lengths | Alternating short (double) and long (single) | All bonds equal length between single and double |
| Reactivity | Typical alkene reactions | Greater thermodynamic stability |
| Electron Density | Localised between 2 atoms | Spread over entire ring |
Visualizing Delocalized Pi Bonds
It helps to think of delocalized bonds as a shared resource rather than a point-to-point link. The classic benzene ring is often represented by a hexagon with a circle inside to symbolize the even electron cloud.
Another metaphor is a pie: if you have two pies but can only eat one, desiring both equally represents the idea of electrons being shared among many atoms rather than fixed to one bond.
Electron Density and Delocalization
Electron density in delocalized pi bonds is “smeared out” across multiple atoms rather than confined. This smearing leads to equal partial double bond characteristics among all bonds in a conjugated system.
Conjugated systems—structures with alternating single and double bonds—allow pi electrons to overlap extensively, increasing the area over which electrons spread.
Summary of Key Points
- A delocalized pi bond involves pi electrons shared across multiple atoms, not fixed between two atoms.
- Common in aromatic compounds, especially benzene, where pi orbitals overlap in a ring to form a continuous electron cloud.
- Resonance structures illustrate the movement of pi bonds, demonstrating electron delocalization.
- Electron delocalization leads to equal bond lengths and increased molecular stability.
- Electron density is spread across the conjugated atoms, lowering energy and reducing reactivity.




Leave a Comment