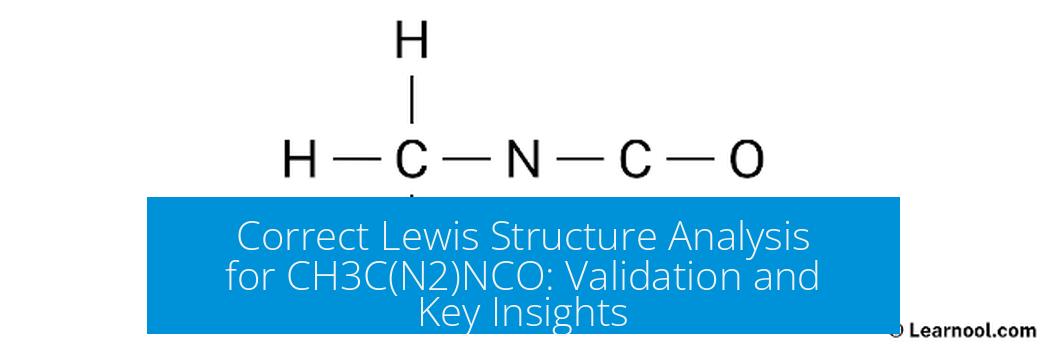
Is this the correct Lewis structure for CH3C(N2)NCO?
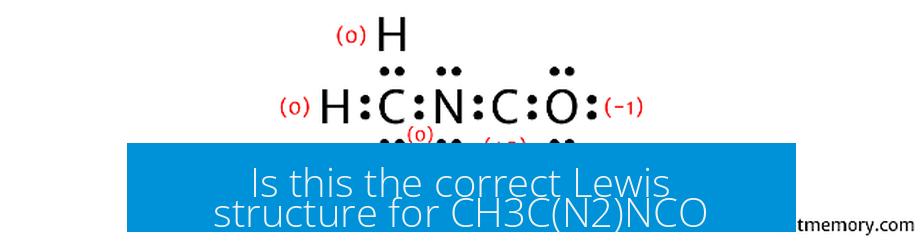
The Lewis structure traditionally assigned to CH3C(N2)NCO is not entirely correct if it places the negative charge incorrectly or misassigns bonding, particularly around nitrogen and oxygen atoms. The correct structure shows the negative charge localized on the oxygen atom within the cyanate moiety, and the nitrogen atoms bonded with appropriate single and double bonds consistent with valence electron counts and formal charge minimization.
Understanding the Formula and Components
The compound CH3C(N2)NCO contains several functional groups: a methyl group (CH3), a diazo group (N2), and a cyanate group (NCO). The structure intertwines these groups, demanding precise electron accounting for correct representation.
- CH3: methyl group with carbon bonded to three hydrogens.
- N2: diazo group containing two nitrogen atoms linked typically by a double or triple bond.
- NCO: cyanate group, which can be represented by different resonance forms, crucially involving the position of charges.
Each section contributes valence electrons to the total count, critical for applying the octet rule and verifying formal charges.
Steps to Validate the Lewis Structure
1. Count Valence Electrons
Sum the valence electrons of each atom:
| Atom | Number | Valence Electrons per Atom | Total Electrons |
|---|---|---|---|
| Carbon (C) | 2 | 4 | 8 |
| Hydrogen (H) | 3 | 1 | 3 |
| Nitrogen (N) | 3 | 5 | 15 |
| Oxygen (O) | 1 | 6 | 6 |
| Total Valence Electrons | 32 |
Confirm if the molecule is neutral or ionic; CH3C(N2)NCO is typically neutral in this context.
2. Draw Skeletal Structure

The logical skeletal structure places the methyl group carbon bonded to the diazo nitrogen, which connects to the cyanate group. Connect atoms focusing on electronegativity and valence preferences.
Example connectivity:
- CH3–C–N≡N (diazo group)–N–C–O (cyanate group)
3. Assign Electrons: Lone Pairs and Bonds
- First, form bonds between atoms using single bonds as a base.
- Add lone pairs to satisfy the octet rule for nitrogen and oxygen atoms, particularly at the periphery.
- Adjust bonds to account for multiple bonds where needed (e.g., N≡N triple bond in diazo).
4. Calculate Formal Charges
Formal charge formula:
(Number of valence electrons in free atom) − (Nonbonding electrons) − 1⁄2 (Bonding electrons)
Calculate for each atom to identify charge distribution. The correct Lewis structure generally minimizes formal charges and places the negative charge on the oxygen atom in the cyanate fragment.
Why the Negative Charge Should Be on Oxygen
Oxygen is more electronegative than nitrogen and carbon. In cyanate ions (NCO−), the most stable resonance structure positions the negative charge on oxygen with appropriate double and single bonds connecting carbon, nitrogen, and oxygen. This aligns with experimental evidence and accepted chemical bonding theories.
- Incorrect structures place the negative charge on nitrogen, which is less favored.
- Correct bonding shows carbon double bonded to oxygen and single bonded to nitrogen.
Special Considerations for Diazo Group

The diazo group (N2) usually exists as a moiety containing a nitrogen-nitrogen triple bond, sharing electrons to complete their octets. This affects electron count calculations in the total molecule.
The bonding around these atoms requires careful double and triple bond assignment, preserving stable octet configurations.
Using Computational Tools for Verification
The Lewis Structure Generator tool aids in confirming the structure by:
- Entering the exact molecular formula “CH3C(N2)NCO”.
- Obtaining a calculated Lewis structure considering valence electrons and bonding preferences.
- Comparing the generated structure with manually drawn ones to spot discrepancies.
This tool saves time and verifies the correctness of complex structures like this molecule.
Multiple Valid Lewis Structures and Resonance
CH3C(N2)NCO can have resonance forms, especially in the cyanate region. However, ideal resonance structures should:
- Follow octet rule except justified exceptions.
- Minimize formal charges and locate negative charges on electronegative atoms like oxygen.
- Maintain charge neutrality or proper charge placement as per molecule’s net charge.
Assessing these factors picks the most plausible Lewis structure among candidates.
Molecular Representation and Limitations
Remember, Lewis structures are two-dimensional representations. They do not show molecular shape or polarity directly, but depict connectivity and electron arrangements.
Use them as guides for further analysis such as predicting molecular geometry with VSEPR theory or computational chemistry methods.
Summary of Verification Strategy for CH3C(N2)NCO Lewis Structure
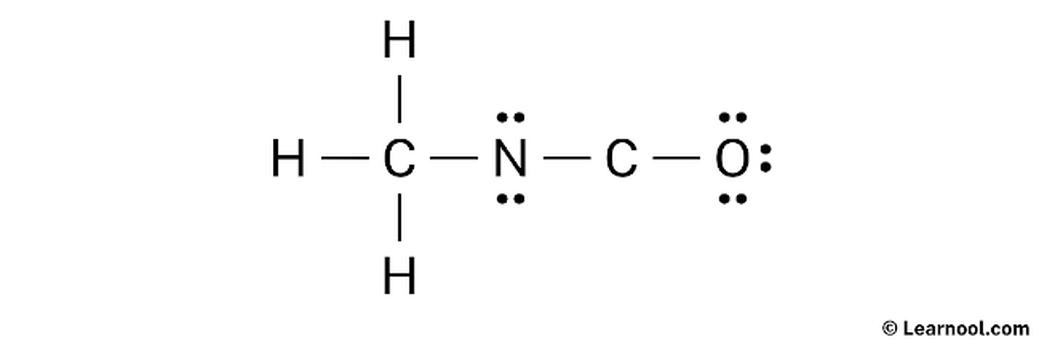
- Accurately count total valence electrons from all atoms.
- Design skeletal connections reflecting known bonding patterns (CH3, diazo, cyanate).
- Assign electrons to satisfy octet rules for second-period atoms.
- Calculate formal charges across all atoms; choose structure minimizing formal charges.
- Place negative charges on the most electronegative atoms, especially oxygen in cyanate.
- Use Lewis Structure Generator tools for quick comparison and validation.
- Consider resonance but rely on chemical intuition and stability principles.
Key Takeaways
- The common Lewis structure assigning the negative charge incorrectly on nitrogen in CH3C(N2)NCO is mistaken.
- The correct Lewis structure places the negative charge on oxygen within the cyanate part.
- Formal charge calculation and octet rule compliance ensure structure accuracy.
- Computational tools can expedite confirmation for complex molecules.
- Diazo and cyanate functional groups require careful bonding assignments reflecting their known chemistry.
- Lewis structures represent connectivity but do not provide full molecular geometry.
Is This the Correct Lewis Structure for CH3C(N2)NCO?
Short answer: No, the common Lewis structure people first draw for CH3C(N2)NCO often isn’t quite right. The key lies in where the negative charge settles and how bonds arrange, especially around the nitrogens and the last carbon and oxygen.
So you’ve stumbled upon the formula CH3C(N2)NCO and asked yourself, “Is this my correct Lewis structure?” Let’s break it down, demystify those atoms, and check whether that skeletal sketch really holds water—or electrons—in the right places.
Using the Lewis Structure Generator Tool: The Quick Check
Before you get elbow-deep in pencil sketches, you could use a nifty Lewis Structure Generator Tool. This software lets you plug in the exact formula—CH3C(N2)NCO—and magically churns out the corresponding Lewis structure. It’s like a molecular cheat sheet.
This saves time and gives you an automated verification. Is the structure you drew before the same as this? If not, you have grounds to investigate further. But remember: the tool is only as good as the formula input and the general rules it follows.
Manual Steps to Get it Right: Counting Electrons and Drawing the Scaffold
Don’t toss away your pencil just yet! Manually drawing a Lewis structure remains a vital skill. Here’s how you tackle CH3C(N2)NCO:
- Count all valence electrons carefully. Carbon has 4 each, nitrogen 5, oxygen 6, hydrogen 1. Add them up, minding any charges or radicals.
- Draw the skeletal structure. Usually, the least electronegative atoms take central spots. For CH3C(N2)NCO, carbon sits at the center linking the groups logically: methyl (CH3), diazo (N2), and cyanate (NCO).
- Add lone pairs to peripheral atoms. Hydrogens stick with two electrons, nitrogen and oxygen get their octets filled as best as possible.
- Complete octets for central atoms while respecting the octet rule unless you justify exceptions (heavier atoms, etc.).
- Count electrons in bonds and lone pairs again. Their sum should match the valence electron count to validate the arrangement.
Making a mistake at any step here spells structural chaos.
Some Atoms Don’t Play by the Rules: When Multiple Lewis Structures Surface
Here’s where CH3C(N2)NCO gets tricky—not just one correct Lewis structure might exist. Resonance structures, tautomerism, or just drawing quirks can yield multiple versions, each plausible but not identical.
How to choose? Use formal charge calculations.
| Atom | Valence Electrons (V) | Nonbonding Electrons (N) | Bonding Electrons (B) | Formal Charge = V – (N + B/2) |
|---|---|---|---|---|
| Carbon | 4 | 0 (if fully bonded) | 8 | 4 – (0 + 8/2) = 0 |
| Nitrogen | 5 | 2 (one lone pair) | 6 (three bonds) | 5 – (2 + 6/2) = 0 |
| Oxygen | 6 | 6 (three lone pairs) | 2 (one bond) | 6 – (6 + 2/2) = -1 |
In short, a stable Lewis structure often places any negative charge on the most electronegative atom—oxygen in this case—to minimize formal charges overall. So if your sketch shows the negative charge perched awkwardly on nitrogen, it’s a red flag.
What About the Last Carbon and Charge Distribution?
The carbon at the tail end of this molecule (in the NCO group) really demands attention. It forms part of the cyanate ion subunit (NCO-), and how it bonds with nitrogen and oxygen affects the entire molecule’s stability.
- The cyanate ion typically features a triple bond between nitrogen and carbon and a double bond (or resonance double-single) between carbon and oxygen.
- The oxygen tends to carry the negative charge, stabilizing the structure.
- When you see single bonds surrounding that carbon instead of the proper multiple bonds, the octet might not be complete or formal charges misassigned.
The lesson? Check the last carbon’s bonding and the charge placement carefully. An incorrect Lewis structure usually misplaces bonds or charges here.
The Bottom Line on CH3C(N2)NCO’s Lewis Structure
To recap, here’s what you want your Lewis structure to do:
- Respect the octet rule for second-period elements.
- Put negative charges on the most electronegative atoms.
- Minimize formal charges across the molecule.
- Have a logically connected skeletal structure that reflects the molecule’s true atomic connections.
- Represent the cyanate ion (NCO-) subunit realistically, which is well studied.
Any structure for CH3C(N2)NCO that fails on these points can be rejected.
Still Stumped? Here’s How to Know If You Got It Right
- Use a Lewis structure generator to get a base reference.
- Count your valence electrons and ensure the number of electrons in bonds and lone pairs adds up.
- Calculate formal charges for each atom. Aim for minimal total formal charge and negative charges on oxygens.
- Examine the cyanate (NCO-) group structure. Does it resemble validated resonance structures?
- Think about octet completion and skeletal connectivity.
- Adjust bonds or lone pairs until the previous conditions are met.
Once all checks out, congratulations, you have your correct Lewis structure!
Why Should You Care? The Bigger Picture
Don’t think Lewis structures are just for passing tests. They lay the groundwork for predicting molecular shape, understanding reactivity, and determining polarity and charge distribution. For CH3C(N2)NCO, getting this right helps chemists comprehend how it might interact in reactions, stability zones, or when incorporated into materials or drugs.
Still not convinced? Think about diazo compounds, to which the N2 moiety belongs. Their unique bonding demands precision. Misplacing charges or bond orders can lead you severely astray when predicting their properties.
Have a Go Yourself!
Grab pen and paper or your trusty digital tool. Try sketching the Lewis structure of CH3C(N2)NCO. Start with the methyl group, add the diazo group, then the cyanate. Count electrons. Calculate formal charges. Adjust. Repeat.
When you get it right, you’ll feel a lot like a detective solving mysteries at the atomic level. You might even surprise yourself at how satisfying it is.
Conclusion
The short version? The commonly drawn Lewis structure for CH3C(N2)NCO is not correct if it places the negative charge wrongly or misses proper bonding in the cyanate ion fragment. Using formal charge calculation, electron counting, and thoughtful skeletal drawing confirms the right arrangement—where oxygen typically carries the negative charge, bonds are properly ordered, and the molecule obeys octet rules or their justified exceptions.
So, next time you wonder about your Lewis structure for CH3C(N2)NCO, pull out your electron-counting hat, grab a structure generator tool for help, and keep an eye on formal charges and octets. That’s chemistry detective work at its best.
What is the best way to verify the Lewis structure of CH3C(N2)NCO?
Use a Lewis Structure Generator tool by entering the molecule’s formula. It quickly produces a correct structure to compare with yours. This helps confirm if your manual drawing matches the expected arrangement.
How do I know if the Lewis structure for CH3C(N2)NCO is correct in terms of electrons?
Count all valence electrons accurately, then draw the skeletal structure. Assign lone pairs and bonds so each atom meets octet rules where applicable. Ensure the total electron count equals the sum of valence electrons for all atoms.
Why are formal charges important when checking CH3C(N2)NCO Lewis structures?
Formal charges help identify the most plausible structure. The correct Lewis structure minimizes formal charges and places negative charges on more electronegative atoms, like oxygen in this molecule.
Is the initial Lewis structure with a negative charge on nitrogen correct for CH3C(N2)NCO?
No, the negative charge should be on the oxygen atom. The structure with proper single and double bonds around nitrogen and the charge on oxygen fits better chemically.
Can CH3C(N2)NCO have multiple valid Lewis structures?
Yes, it can have resonance forms. You must assess which structure obeys octet rules better and has minimal formal charges. The structure that best fits these criteria is considered correct.




Leave a Comment