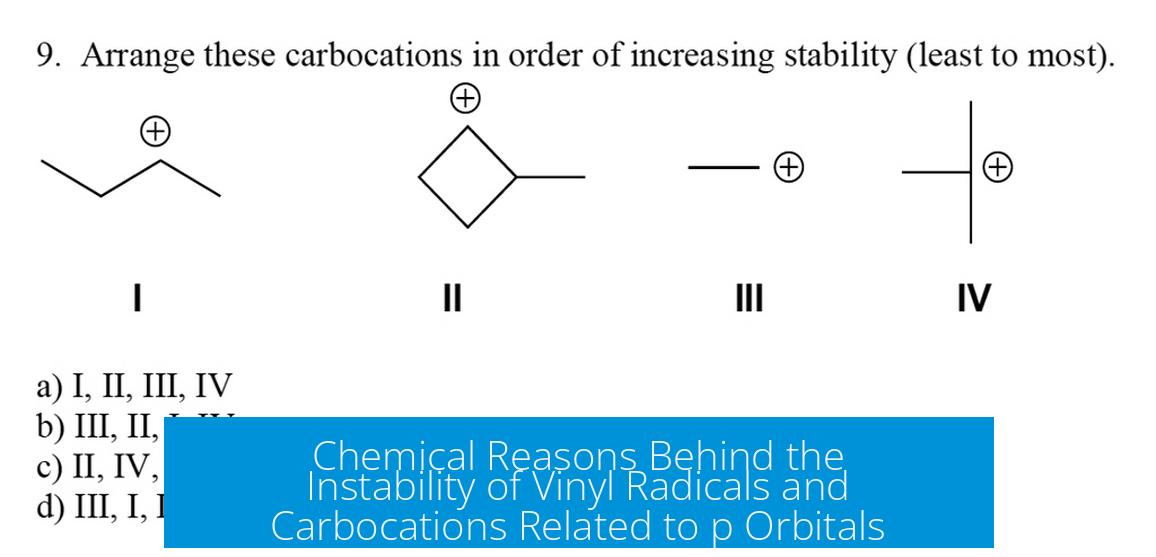
Chemical Explanation for the Instability of Vinyl Radicals and Carbocations
Vinyl radicals and carbocations are unstable primarily because of their sp2 hybridization, which affects the electron deficiency and orbital s-character at the carbon bearing the charge or radical.
Hybridization and s-Character
Vinyl carbons have an sp2 hybridization. This means their atomic orbitals consist of one s orbital and two p orbitals combined, leaving one unhybridized p orbital.
The s-character of an orbital determines how close the electron deficiency lies to the nucleus. More s-character usually means orbitals are closer to the nucleus.
Impact of s-Character on Stability
Orbitals with higher s-character hold electron density closer to the nucleus, which influences stability. For common carbon hybridizations:
- sp3 orbitals have 25% s-character and are furthest from the nucleus.
- sp2 orbitals have 33% s-character, positioning the electron deficiency closer.
- sp orbitals have 50% s-character, closest to the nucleus.
Since vinyl carbons are sp2 hybridized, their positive charge or radical sits closer to the nucleus than in sp3 hybridized centers. This proximity generally destabilizes the charged or radical site.
Why Vinyl Radicals and Carbocations Are Less Stable
Vinyl radicals and carbocations are electron-deficient species formed at sp2 carbons. The greater s-character makes the positive charge or unpaired electron closer to the nucleus, increasing electron deficiency and lowering stability.
In contrast, sp3 alkyl radicals or carbocations spread their electron deficiency over orbitals with lower s-character, which are further from the nucleus, resulting in greater stability.
Role of p Orbitals
While vinyl carbons have an unhybridized p orbital, the instability does not directly arise from the presence of p orbitals. Instead, the sp2 hybridization and s-character govern how the charge interacts with the nucleus, affecting stability.
Summary of Key Points
- Vinyl carbons are sp2 hybridized, with 33% s-character in bonding orbitals.
- Greater s-character brings charges or radicals closer to the nucleus, destabilizing them.
- Sp2 hybridization results in less stable vinyl radicals and carbocations compared to sp3 analogs.
- Unhybridized p orbitals exist but do not directly cause instability here.



Leave a Comment