Understanding the Multiplicity of Proton NMR for Biphenyl
The multiplicity of a proton NMR signal for biphenyl is generally described as a multiplet. This is primarily due to the complexity of the aromatic proton environment in biphenyl and the resulting overlapping splittings. Sometimes, the multiplicity can resemble a doublet of triplets, but overall, calling the peaks a multiplet best captures the spectral features without oversimplification.
What Is Multiplicity in Proton NMR?
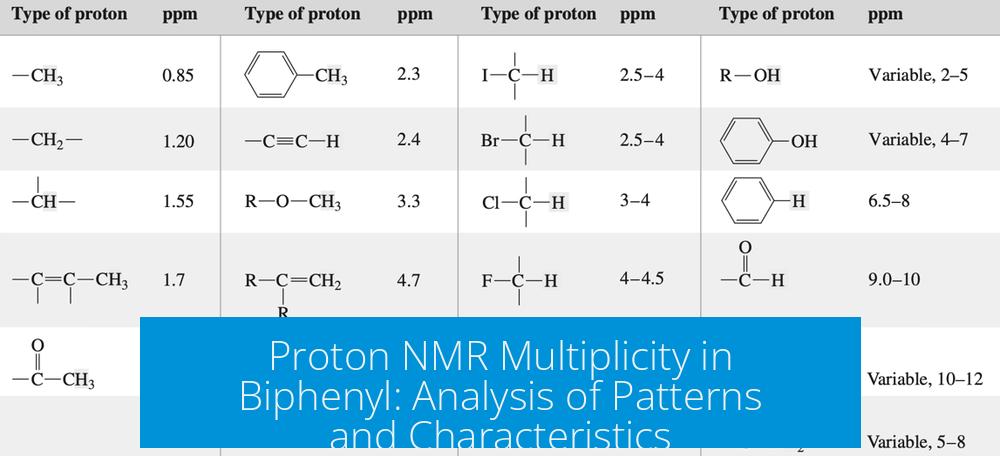
Multiplicity refers to the splitting pattern of NMR signals caused by spin-spin coupling with neighboring nuclei. Simple alkyl protons often show well-defined doublets, triplets, or quartets based on the number of adjacent protons. However, aromatic systems like biphenyl involve more complex coupling networks.
Why Biphenyl Proton NMR Multiplicity Is Complex
Biphenyl contains two phenyl rings linked by a single bond. Each ring has several aromatic protons that influence each other’s NMR signals through scalar coupling. This results in overlapping signals and poor resolution of clear splitting patterns.
- Protons experience coupling with adjacent (ortho), nearby (meta), and sometimes longer-range protons.
- Second order effects in aromatic systems distort the expected splitting patterns, bending triplets and doublets from ideal shapes.
- The coupling constants (J values) may vary subtly between protons, which makes manual analysis challenging.
- Protons can be magnetically non-equivalent, even when chemically equivalent, due to the ring environment.
Typical Multiplicity Patterns Observed in Biphenyl
The most recognizable pattern inside the aromatic region for biphenyl is a multiplet. This term encompasses complex overlapping signals without precisely resolved smaller splittings.
Researchers sometimes describe biphenyl signals as a doublet of triplets (dt). This is not always exact, but the appearance of the peaks can resemble this pattern, affected by:
- Couplings with ortho and meta protons.
- Small coupling constants overlapping each other.
- Peak ratios distorted by overlapping signals and second order effects.
Symmetry Effects in Biphenyl Proton NMR
Biphenyl symmetry reduces the number of unique signals. Four distinct aromatic proton signals can simplify into effectively two sets due to symmetrical phenyl rings.
- Ortho protons on one phenyl ring are homotopic (chemically identical) and share the same chemical shift.
- Despite this, these protons are not magnetically equivalent, because their coupling partners differ.
- Symmetry reduces spectral complexity but does not eliminate overlapping and complex multiplicity.
Challenges in Analysis
Aromatic protons in biphenyl produce spectra that are often too complicated for first-order manual analysis. Overlapping peaks mask smaller splittings and coupling constants.
The coupling constants cannot be reliably extracted without more advanced spectral simulations or experiments. Therefore, spot assignments such as “doublet of triplets” remain tentative without J-value confirmation.
Some specific difficulties:
- Second order effects cause peaks to lean and lose ideal triplet or doublet shapes.
- Peak distortions cause loss of smaller coupling peaks, changing intensity ratios.
- Some expected peaks may be missing or hidden in shoulders of larger peaks.
Best Practices for Analysis and Reporting
Given these complexities, standard practice typically involves reporting the proton signals associated with biphenyl aromatic protons as multiplets.
- Use the term “multiplet” without assuming ideal splitting.
- Zoom in on spectra to resolve peak shape and count integrals for proton number confirmation.
- Attempt spectrum prediction and overlay to better understand possible split patterns.
- Consider diluting samples to reduce peak overlap and baseline noise when possible.
- Utilize 2D NMR methods for improved assignment, such as COSY or NOESY.
Summary of Multiplicity Characteristics for Biphenyl Proton NMR
| Aspect | Description |
|---|---|
| Multiplicity Type | Generally described as a multiplet; often resembles doublet of triplets |
| Coupling Partners | Ortho and meta protons on aromatic rings; two-bond and three-bond couplings |
| Symmetry Effect | Reduces four signals into two sets due to homotopic protons |
| Challenges | Peak overlap, second order effects, distorted intensities, missing small peaks |
| Practical Reporting | Report as multiplet unless detailed coupling constants are confirmed |
Additional Insights
While simplicity is often recommended by calling it a multiplet, spectral specialists sometimes refine the description when careful simulation or relaxation experiments can reveal coupling constants. When forced to assign precise multiplicity, doublet of triplets is a reasonable hypothesis.
Ignoring complexity is tempting but not advised if the experiment demands detailed structural information or if isotopic labeling or substituted analogs provide clearer signals.
Key Takeaways
- Biphenyl proton NMR signals are usually reported as multiplets due to complex aromatic coupling.
- The multiplicity pattern often resembles a doublet of triplets but is obscured by overlapping and second order effects.
- Symmetry reduces signal number but not complexity.
- Manual assignment is difficult; use simulation and higher resolution methods if detailed splitting is required.
- Practical spectral analysis prefers calling it a multiplet unless J-coupling data justify further detail.




Leave a Comment