Mechanism for Creating 1-Butene from 2-Chlorobutane

The formation of 1-butene from 2-chlorobutane occurs through an elimination reaction, specifically an E2 mechanism facilitated by a sterically hindered base such as potassium t-butoxide. This base selectively removes the proton from the terminal carbon, leading to the formation of 1-butene over other possible isomers.
Elimination Reaction Overview
2-Chlorobutane contains a chloride leaving group at the second carbon. In the presence of a strong base, elimination reactions proceed by removing a proton (β-hydrogen) adjacent to the carbon bearing the leaving group. The base abstracts this proton, the leaving group departs, and a double bond forms.
Role of Sterically Hindered Base
- Potassium t-butoxide (KOtBu) is a bulky base, hindering its approach to crowded sites.
- This steric bulk favors abstraction of the most accessible β-proton, commonly at a less substituted or terminal carbon.
- By contrast, smaller bases might abstract protons less selectively, leading to a mixture of alkenes.
Selectivity for 1-Butene Formation
In 2-chlorobutane, β-hydrogens exist on both the first and third carbons. The bulky base preferentially abstracts the proton on the primary (terminal) carbon rather than the secondary (internal) carbon. This preference promotes the formation of the less substituted alkene: 1-butene.
The selective proton abstraction is crucial because it guides the elimination towards the terminal double bond despite Zaitsev’s rule, which otherwise favors more substituted alkenes.
Mechanistic Steps
- Potassium t-butoxide approaches 2-chlorobutane.
- The bulky base abstracts the terminal β-proton (on the first carbon).
- Simultaneously, the chloride ion leaves from the second carbon.
- A double bond forms between the first and second carbons, yielding 1-butene.
Summary of Factors Favoring 1-Butene
| Factor | Effect |
|---|---|
| Bulky base (KOtBu) | Favours abstraction of accessible β-protons |
| Proton location | Terminal β-protons more accessible than internal |
| Simultaneous E2 mechanism | Concerted proton abstraction and leaving group departure |
In summary, the use of a sterically hindered base induces selective elimination at the terminal position of 2-chlorobutane, producing 1-butene with high selectivity.
Key Takeaways
- 1-Butene forms from 2-chlorobutane via E2 elimination with a bulky base.
- Potassium t-butoxide selectively removes terminal β-protons due to steric hindrance.
- This selectivity overrides Zaitsev’s rule, favoring the less substituted alkene.
- Simultaneous proton abstraction and chloride exit yield 1-butene efficiently.
Mechanism for Creating 1-Butene from 2-Chlorobutane
So, you want to turn 2-chlorobutane into 1-butene? The key here is a sterically hindered elimination reaction. This means the choice of base matters a lot. You don’t want just any base—you want a bulky one that knocks off the right proton to get 1-butene instead of 2-butene. Let’s break this down.
Why Does the Base Size Matter?
Imagine a crowded party. You’re the bulky base, and you’re trying to grab a proton. The internal protons are surrounded by other groups—basically, it’s packed tight. The terminal proton, on the other hand, is hanging out at the edge of the dance floor, looking easy to reach. Naturally, the bulky base goes for the “easy-to-grab” terminal proton.
This is why potassium t-butoxide shines here. It’s a big, bulky base. It can’t squeeze around the tight places near the internal carbons. So, it hits the terminal proton, triggering elimination that yields 1-butene. Smaller bases don’t show this selective behavior—they can sneak in and grab internal protons, giving you a mix of products. Using potassium t-butoxide ensures better selectivity for forming 1-butene over 2-butene.
Mechanism: Step-by-step
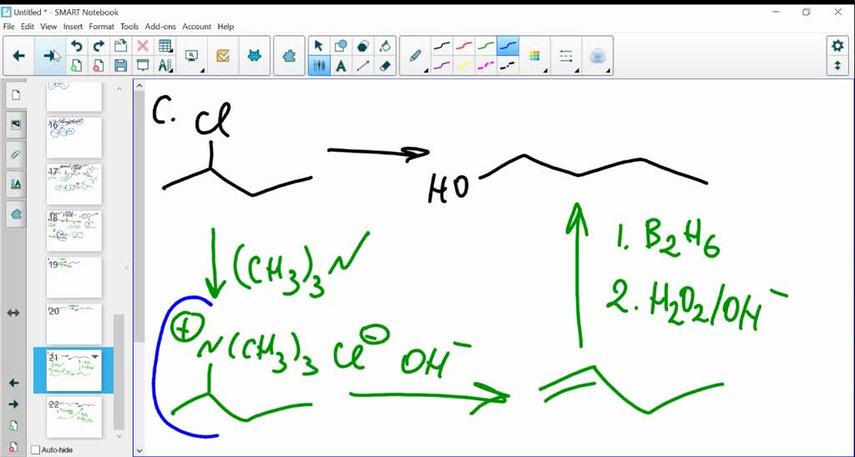
- Start with 2-chlorobutane, which has a chlorine attached to the second carbon.
- Potassium t-butoxide approaches. Because it’s bulky, it avoids the crowded second carbon region.
- It abstracts a proton from the terminal carbon (carbon 1), which is more accessible.
- The elimination proceeds via an E2 mechanism—proton departure and leaving group (chloride) elimination happen simultaneously.
- Double bond forms between carbon 1 and carbon 2, producing 1-butene.
Because the base attacks the terminal proton, you avoid forming the more substituted but less desired 2-butene. This is a textbook example of steric control steering elimination products.
Is the Process Perfectly Selective? Not Quite.
One might wonder, “Is potassium t-butoxide always picky enough?” The short answer is no. There’s some concern about selectivity when dealing with butane derivatives. The base might sometimes poll the internal protons, producing 2-butene as a side product. However, the bulkiness of potassium t-butoxide gives it a clear preference. The terminal proton gets knocked out most of the time.
Think of it like pecking order at the proton party. The bulky base is best attuned to the easiest target first, but if it’s feeling extra ambitious, it might go for others too. Still, the majority product remains 1-butene.
Why Use Large, Bulky Bases at All?
Why not just use any base? The big lesson from organic chemistry is about controlling reaction outcomes. Using bulky bases gives chemists control over which alkene forms. This matters because 1-butene and 2-butene have different properties and reactivities: 1-butene is terminal, 2-butene is internal.
Terminal alkenes like 1-butene are often more reactive in polymerization and other reactions. So, if you want to make specialty plastics or need specific intermediates, getting that 1-butene selectively is important. Using potassium t-butoxide to favor 1-butene saves you from messy mixtures and tedious separations.
Practical Tips & Examples for Lab Enthusiasts

- Keep the base bulky: Potassium t-butoxide is your go-to. Smaller bases like hydroxide or ethoxide increase the chance of internal proton removal, making your product mixture more complicated.
- Use a non-polar solvent: Solvents like tert-butanol or ether help maintain the bulky base’s shape and favor E2 elimination.
- Temperature control matters: Higher temperatures typically favor elimination. But excessive heat might generate side reactions—keep the balance.
- Watch out for rearrangements: With 2-chlorobutane, you usually avoid carbocation intermediates in E2. But if conditions are wrong, side reactions may occur.
What Happens If You Use the Wrong Base?
Imagine swapping potassium t-butoxide for sodium hydroxide. Sodium hydroxide is smaller and not as bulky. It can access internal protons more easily. The result? A mix of 1-butene and 2-butene. Sometimes the more substituted 2-butene dominates because it’s more stable thermodynamically. So, you lose selectivity. The reaction becomes less predictable and less efficient.
In conclusion, bulkiness equals control. Keep your base bulky and sterically demanding to guide the elimination toward 1-butene.
Summing It Up
To craft 1-butene from 2-chlorobutane, chemists rely on steric hindrance. Potassium t-butoxide, a bulky base, selectively strips off the terminal proton during an E2 elimination. This avoids internal proton abstraction that leads to 2-butene. While the process isn’t perfectly selective, this method remains the best strategy to favor 1-butene.
So next time you want that terminal alkene, remember—size matters, and potassium t-butoxide has you covered.
What type of elimination mechanism is involved in forming 1-butene from 2-chlorobutane?
The reaction uses a sterically hindered elimination mechanism. A bulky base abstracts a proton to favor forming 1-butene over other isomers.
Why is potassium t-butoxide preferred as the base in this reaction?
Potassium t-butoxide is large and sterically hindered. This size helps it selectively remove terminal protons, leading to the formation of 1-butene.
How does the base selectivity influence the product distribution?
The bulky base favors elimination of a proton from the terminal carbon. This selectivity reduces formation of internal alkenes and promotes 1-butene production.
Could other bases work as well to produce 1-butene?
Other bases may not be as selective. Smaller bases may remove protons from different positions, giving a mix of products. Large, hindered bases work best.
Is the elimination reaction always selective for 1-butene?
Not always. Steric hindrance increases selectivity, but some internal proton abstraction can still happen. The choice of base is key in enhancing selectivity.




Leave a Comment