How Do I Make HCl?
Hydrochloric acid (HCl) is a highly corrosive chemical best obtained through purchase rather than synthesis due to its dangers and legal restrictions. Producing HCl involves risks and should only be performed by trained professionals with proper safety measures. This article outlines methods and important considerations for handling HCl.
Legal and Safety Considerations
Making HCl without proper training is unsafe and illegal in many places. HCl is regulated because it can be used to produce harmful substances. Proper protective equipment (PPE) and ventilation are mandatory during any approved chemical procedures.
Purchasing Hydrochloric Acid
- Muriatic acid, a commercial form of HCl, is available at hardware and pool supply stores. It is typically around 8 to 10 Molar concentration.
- Reagent-grade HCl is stronger (up to 12 M) but may require special suppliers like chemical companies.
- Small quantities are also sold online from verified vendors.
For most uses, muriatic acid serves well despite possible minor impurities.
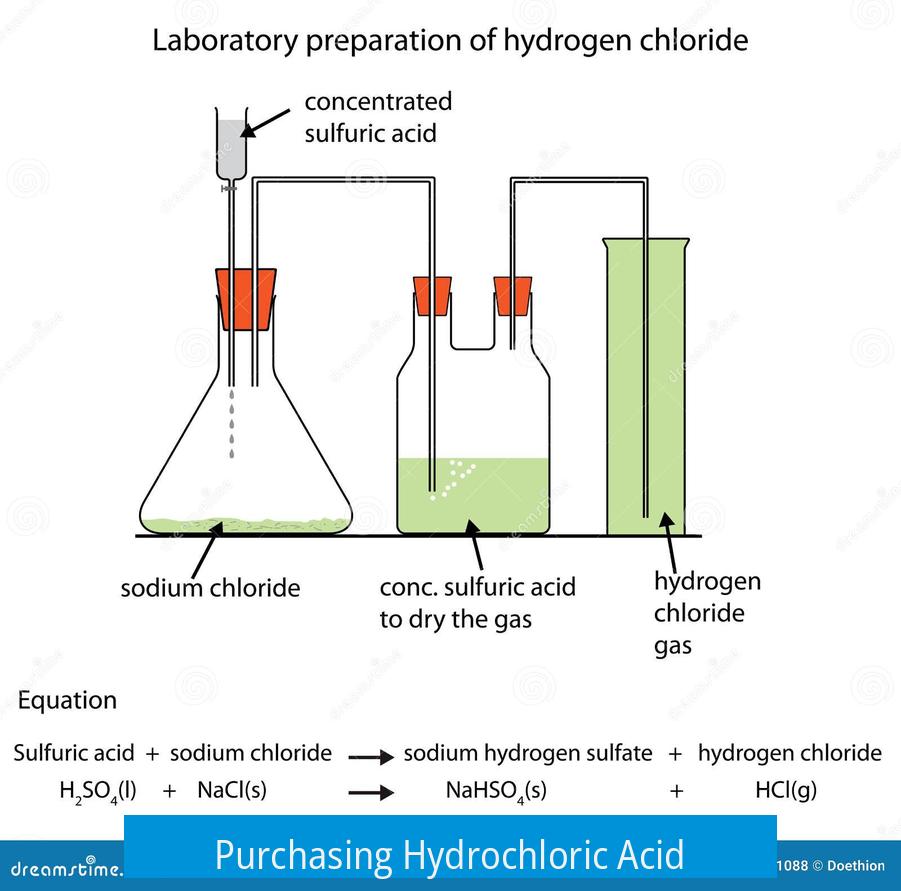
Laboratory Synthesis Method
One standard lab synthesis combines sodium chloride (table salt) with concentrated sulfuric acid. The reaction produces hydrogen chloride gas:
NaCl(s) + H2SO4(l) → HCl(g) + NaHSO4(s)
This HCl gas can be bubbled into water to form hydrochloric acid solution. The process requires:
- A flask to contain the sodium chloride.
- Dripping concentrated sulfuric acid carefully into the flask.
- Collecting the HCl gas via a sidearm.
- Absorbing the gas into water, but avoiding water backflow into the reaction flask.
The handling of corrosive fumes and reactive substances mandates full PPE and proper ventilation.
Purification via Distillation

To obtain pure hydrochloric acid, one may distill the aqueous solution. HCl forms an azeotrope with water around 6 M concentration, limiting purification by simple distillation.
Alternative Considerations
Electrolysis methods exist but require complex setups not suitable for amateur chemistry. Always prioritize safety and legality when handling or preparing HCl.
Summary of Key Points
- Producing HCl is hazardous and often illegal without licenses.
- Buying muriatic acid is the safest and most practical approach.
- Lab synthesis via sodium chloride and sulfuric acid releases toxic HCl gas.
- Proper PPE, ventilation, and strict control are essential in synthesis.
- HCl purification is limited by azeotropic behavior during distillation.
How Do I Make HCl? A Detailed Look at Hydrochloric Acid Preparation
If you’re asking, “How do I make HCl?” here’s the straightforward answer: It’s complicated, risky, and often illegal to try synthesizing hydrochloric acid yourself without proper training and safety equipment. Hydrochloric acid (HCl) is a powerful, corrosive chemical used extensively in industry and laboratories. But whipping up HCl at home or without expertise isn’t something to take lightly. Let’s unpack this, including what you need to know about making it, buying it, and staying safe.
Why Not Just Make It Yourself?
Here comes the tough love first: synthesizing hydrochloric acid isn’t a home DIY project. HCl is a controlled substance. Why? It can be misused to make illegal drugs or cause harm. Also, the process involves hazardous chemicals that release corrosive fumes. You need specialized training and equipment to handle it safely.
Already curious about how it’s done? Great! But always wear personal protective equipment (PPE), work in a fume hood or very well-ventilated area, and be aware of the risks. Otherwise, just buy it. More on that soon.
Buying Hydrochloric Acid: The Safe Shortcut
The easiest and most sensible way to get HCl is to buy it. If you walk into a hardware store, you’ll find muriatic acid, which is a commercial name for hydrochloric acid.
- Muriatic acid is usually around 8 to 10 molar (M), less concentrated than lab-grade (12 M) reagents.
- It’s cheap, often less than $10 per gallon.
- Pool supply stores also stock it for pool pH adjustments.
For lab work, brands like Millipore Sigma sell bioreagent grade HCl in 100 mL bottles, which are more pure but pricier and require careful handling.
Buying from hardware or pool stores is generally fine for cleaning or general use. Just remember, muriatic acid may have impurities you don’t want if your application needs high purity.
The Classic Chemistry Trick: Making HCl Gas from Salt and Sulfuric Acid
For chemistry buffs with the right skills and setup, making HCl gas from table salt (sodium chloride, NaCl) and sulfuric acid (H2SO4) is the classic reaction. This method is old-school but effective:
NaCl (solid) + H2SO4 (liquid) → HCl (gas) + NaHSO4 (solid)
The reaction produces anhydrous HCl gas, which you then bubble through water to create hydrochloric acid solution. But heads up: This process is highly corrosive, releases dangerous fumes, and must be done in a fume hood with PPE like gloves, goggles, and a lab coat.
The technique involves:
- Placing solid table salt in a flask.
- Dripping sulfuric acid slowly into the flask through a stopper with a tube.
- Collecting the escaping HCl gas via the sidearm tube.
- Carefully bubbling the gas into water in a separate container.
Important safety tip: Never let the tube end dip underwater. Water will siphon back into the reaction flask and cause dangerous splashing. Also, as HCl dissolves, the solution volume increases, so use a big enough container.
This method is educational but too risky for untrained amateurs.
Cleaning It Up: Purification by Distillation
After generating hydrochloric acid, you might want to purify it. HCl forms an azeotrope with water at about 6 M concentration and boils at a constant temperature. Distillation can separate HCl from impurities. This is common in labs but requires specialized glassware and skills.
So yes, if you aim for pure acid, distillation is your friend. But it’s a step better left to pros.
Other Possibilities and Notes
Some chemistry textbooks mention electrolysis methods to produce hydrochloric acid, though these are more complex. Also, some people might wonder if a small animal can dissolve in HCl—but that’s a different story with ethical considerations.
Not all countries sell hydrochloric acid freely; geographic laws can block access. So sometimes buying muriatic acid locally isn’t an option. That pushes some to consider synthesis, but again, caution is THE rule.
Final Thoughts: Should You Try Making Hydrochloric Acid?
Here’s the nutshell: If you don’t have training, proper lab setup, or legal clearance, you really shouldn’t try to make hydrochloric acid yourself.
Buying muriatic acid from a store is safe, affordable, and suitable for most non-lab purposes. For scientific needs, buy reagent-grade from trusted suppliers.
If you want to experiment with making HCl gas from salt and sulfuric acid, learn all about safe lab practices first. That means PPE, ventilation, emergency protocols, and expert supervision.
There’s no shortcut around safety when dealing with something this potent. Ever heard the phrase, “Better safe than sorry”? It applies here big time.
Some Handy Takeaways
- Muriatic acid is your easy, affordable source of hydrochloric acid.
- Never try homemade synthesis without proper training and safety gear.
- If synthesizing, use sodium chloride and sulfuric acid carefully in a controlled lab setting.
- Purify hydrochloric acid by distillation only if you have the knowledge and equipment.
- Always follow local laws and regulations regarding acid handling and acquisition.
So next time you wonder “How do I make HCl?” remember: sometimes the best chemistry is knowing when to buy and when to leave it to the pros. Got questions? Feel free to ask, but first, gear up with safety knowledge before diving into any acid adventures!
How can I make hydrochloric acid using common chemicals?
You can react table salt (NaCl) with sulfuric acid. This produces HCl gas, which you must bubble through water to get hydrochloric acid. Handle this with care due to harmful fumes.
Is it possible to safely make HCl at home?
It is not safe without proper chemical knowledge and equipment. Making HCl involves dangerous reactions and toxic gases. Professional training and safety gear are necessary.
Where can I buy hydrochloric acid instead of making it?
Muriatic acid, a form of HCl, is available at hardware or pool stores. It usually contains about 8-10 M concentration, sufficient for many uses but may have impurities.
How do I prevent water from contaminating the reaction when making HCl gas?
Never allow the dip tube delivering HCl gas to go underwater. Water can be sucked back into the reaction flask, causing dangerous splashes or reactions.
Can hydrochloric acid be purified after synthesis?
Yes, HCl can be purified by distillation. It forms a 6 M azeotrope which distills cleanly, separating it from other impurities or water.


Leave a Comment