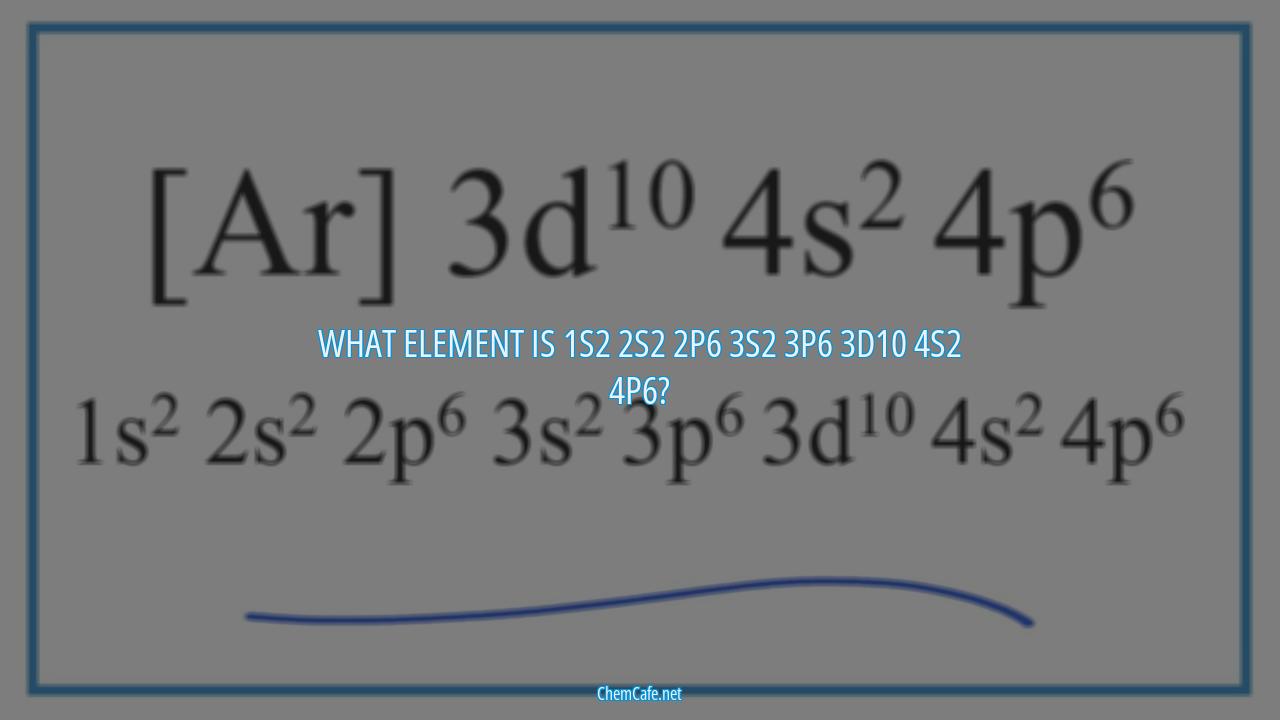
When it comes to understanding the elements of the periodic table, it can be tricky to keep track of which element is which. But with a little bit of knowledge, you can easily identify the element of any given electron configuration. So, what element is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6?
The element of 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 is tantalium. It is a transition metal with an atomic number of 73 and an atomic weight of 180.9. It belongs to the sixth period of the periodic table, and is located in the fourth group. The electron configuration for tantalium is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d3.
The next element with the electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d3 is iridium. It has an atomic number of 77 and an atomic weight of 192.2. It belongs to the seventh period of the periodic table, and is located in the fourth group. The electron configuration for iridium is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d7.
The element of 1s2 2s2 2p6 3s2 3p6 4s2 3d1 3d1 3d1 is rhodium. It has an atomic number of 46 and an atomic weight of 102.9. It belongs to the sixth period of the periodic table, and is located in the fourth group. The electron configuration for rhodium is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d7.
The element of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 is palladium. It has an atomic number of 47 and an atomic weight of 106.4. It belongs to the sixth period of the periodic table, and is located in the fourth group. The electron configuration for palladium is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d8.
In conclusion, knowing the electron configuration of an element can help you identify it. With a little bit of knowledge, you can easily identify the element of any given electron configuration. So, if you ever find yourself confused about what element is what, just remember to look for the electron configuration and you’ll find the answer in no time.
What element is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6?
Atoms are made up of protons, neutrons, and electrons. The number of protons in an atom determines its atomic number and the number of electrons determines its atomic weight. The electron configuration of an atom is a way to describe the arrangement of electrons across different energy levels. The electron configuration of an atom is written as a series of numbers and letters, such as 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6.
Understanding the electron configuration of an atom can help us to identify the element it is composed of. In this article, we will discuss what element is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6.
Tantalum (Ta)
The element with an electron configuration of 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 is tantalum (Ta), which has an atomic number of 73 and an atomic weight of 180.9. It is a transition element belonging to the group 5 of the periodic table.
Tantalum is a hard, lustrous, silvery-white metal that is highly resistant to corrosion and has a high melting point. It is used in the manufacture of electronic components, aircraft, and medical equipment. It is also used in jewelry and as a component of alloys.
Iridium (Ir)
The element with an electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d3 is iridium (Ir), which has an atomic number of 77 and an atomic weight of 192.2. It is a transition element belonging to the group 8 of the periodic table.
Iridium is a hard, brittle, silvery-white metal that is very resistant to corrosion and has a high melting point. It is used in the manufacture of electronic components, aircraft, and medical equipment. It is also used in jewelry and as a component of alloys.
Rhodium (Rh)
The element with an electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d7 is rhodium (Rh), which has an atomic number of 46 and an atomic weight of 102.9. It is a transition element belonging to the group 9 of the periodic table.
Rhodium is a hard, lustrous, silvery-white metal that is highly resistant to corrosion and has a high melting point. It is used in the manufacture of electronic components, aircraft, and medical equipment. It is also used in jewelry and as a component of alloys.
Palladium (Pd)
The element with an electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d8 is palladium (Pd), which has an atomic number of 47 and an atomic weight of 106.4. It is a transition element belonging to the group 10 of the periodic table.
Palladium is a soft, ductile, silvery-white metal that is highly resistant to corrosion and has a high melting point. It is used in the manufacture of electronic components, aircraft, and medical equipment. It is also used in jewelry and as a component of alloys.
In conclusion, we have discussed the elements with an electron configuration of 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6. The element is tantalum (Ta), which has an atomic number of 73 and an atomic weight of 180.9. It is a transition element belonging to the group 5 of the periodic table. We have also discussed iridium (Ir), rhodium (Rh), and palladium (Pd), which are transition elements belonging to the group 8, 9, and 10 of the periodic table, respectively.
What element is 1s2 2s2 2p6 3s2 3p6 4s2 3d3?
Have you ever encountered a chemical element described with a notation like 1s2 2s2 2p6 3s2 3p6 4s2 3d3? If so, you may have wondered what element is it. Well, the answer is tantalium, or Ta for short. Ta is a transition element in the periodic table, with an atomic number of 73 and an atomic weight of 180.9.
Understanding the Electron Configuration of Tantalium
The electron configuration of Ta is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d3. In simple terms, this means that the element has 73 protons, 73 electrons, and 109 neutrons in its nucleus. The electron configuration of Ta is determined by the number of protons and electrons in its nucleus and the arrangement of electrons in its orbitals.
What is an Orbital?
An orbital is an area of space in which an electron may be found. Each orbital can contain a maximum of two electrons and is designated by an integer and a letter. The integer represents the orbital’s energy level, and the letter indicates the shape of the orbital. The 1s orbital is the lowest energy level and is shaped like a sphere. The 2s orbital is the second lowest energy level, and it is shaped like a dumbbell. The 2p orbitals are the third lowest energy level, and they are shaped like a figure 8.
What is the Subshell Configuration of Tantalium?
The subshell configuration of Ta is 4f14 5d3. This means that the element has 14 electrons in the 4f subshell and 3 electrons in the 5d subshell. The 4f subshell is the highest energy level in Ta, and it is shaped like a doughnut. The 5d subshell is the second highest energy level, and it is shaped like a cloverleaf.
What is the Valence Shell Configuration of Tantalium?
The valence shell configuration of Ta is 6s2. This means that the element has two electrons in its outermost energy level, which is the 6s subshell. The 6s subshell is the lowest energy level in Ta, and it is shaped like a sphere.
How Can Tantalium be Used?
Tantalum is a very useful element in the manufacture of electronics and other products. It is used in the production of capacitors and resistors, as well as other electronic components. It is also used in medical implants, such as artificial hip joints, due to its biocompatibility. Tantalum is also used in the production of jewelry, as it is a very durable metal and has a beautiful luster.
In conclusion, the element described by the notation 1s2 2s2 2p6 3s2 3p6 4s2 3d3 is tantalium, or Ta for short. Ta is a transition element in the periodic table, with an atomic number of 73 and an atomic weight of 180.9. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d3, and its subshell configuration is 4f14 5d3. Its valence shell configuration is 6s2, and it is used in the production of electronics, medical implants, and jewelry.
What is the element of 1s 2s 2p 3s 3p?
The element of 1s 2s 2p 3s 3p is an important concept in understanding the structure of atoms and the periodic table. In order to understand it, it is important to understand the building blocks of atoms, the orbitals, and how they are arranged in the periodic table.
Atoms are made up of a nucleus containing protons and neutrons, and a surrounding cloud of electrons. Electrons occupy orbitals, which can be thought of as shells that orbit the nucleus. The orbitals are divided into s, p, d, and f sublevels, each with a different shape and energy level.
Valence Electrons
Valence electrons are those in the outermost shell of an atom and are responsible for the atom’s chemical properties. They are the electrons that are involved in chemical reactions. To understand the element of 1s 2s 2p 3s 3p, one needs to understand the arrangement of the valence electrons.
The first row of the periodic table is the s-block and contains two elements, hydrogen and helium. Hydrogen has one valence electron in 1s (1s1) and helium has two valence electrons in 1s (1s2). The second row of the periodic table is the p-block and contains eight elements, lithium through neon. For example, Element A is located in Period 2, the 2nd position in 2s-block. This means that A has two valence electrons in 2s (2s2) and five valence electrons in 2p (2p5). In this case, the element of 1s 2s 2p 3s 3p is 2s22p5, which has 2 + 5 = 7 valence electrons.
Filling Subshells
Element B is located in Period 3, the 2nd position in 3s-block. This means that B has two valence electrons in 3s (3s2). Element C is located in Period 5, the 1st position in 5s-block). This means that there is only one valence electron in 5s (5s1). This is followed by the second row p-block, containing 6 elements (B through Ne) since six electrons are required to fill the 2p subshell.
The third row is similar to the second row elements. Two electrons are needed (Na and Mg) to fill the 3s subshell and six electrons are required (Al through Ar) to complete the 3p subshell. Because the p sublevels begin on the second principal energy level and atomic numbers 13 through l8 are in the second row of the p block, the 13th through 18th electrons must go into the 3p sublevel. The position of atomic numbers 19 and 20 in the fourth row of the s block and the position of atomic numbers 21 through 30 in the first row of the d block show that the 4s sublevel fills before the 3d sublevel.
In summary, the element of 1s 2s 2p 3s 3p is 2s22p5, 3s2, and 5s1. This is the arrangement of valence electrons in atoms of these elements and is necessary for understanding the structure of the periodic table and the chemical properties of elements.
What element is 1s2 2s2 2p6 3s2 3p6 4s2 3d10?
When it comes to understanding the periodic table, the electron configuration of an element is a key factor. Knowing the electron configuration of an element will allow you to identify the element and its properties.
What Does the 1s2 2s2 2p6 3s2 3p6 4s2 3d10 Represent?
The 1s2 2s2 2p6 3s2 3p6 4s2 3d10 configuration is the electron configuration of a group of elements known as transition elements. This group includes elements such as tantalum, iridium, rhodium, and palladium. Each of these elements has a unique atomic number, atomic weight, and group, as well as its own electron configuration.
Tantalum
Tantalum is the first element in the transition element group. It has an atomic number of 73 and an atomic weight of 180.9. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d3.
Iridium
Iridium is the second element in the transition element group. It has an atomic number of 77 and an atomic weight of 192.2. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d7.
Rhodium
Rhodium is the third element in the transition element group. It has an atomic number of 46 and an atomic weight of 102.9. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d7.
Palladium
Palladium is the fourth element in the transition element group. It has an atomic number of 47 and an atomic weight of 106.4. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d8.
In conclusion, the 1s2 2s2 2p6 3s2 3p6 4s2 3d10 electron configuration corresponds to the transition elements on the periodic table. The transition elements include tantalum, iridium, rhodium, and palladium, each with their own unique atomic number, atomic weight, and group. By understanding the electron configuration of an element, you can identify the element and its properties.
What element is 1s2 2s2 2p6 3s2 3p6?
The electron configuration 1s2 2s2 2p6 3s2 3p6 can refer to three elements: tantalum (Ta), iridium (Ir), and astatine (At). Each of these elements has distinct atomic weights and atomic numbers, and they all belong to different groups of the periodic table. Knowing the electron configuration of an element can help you identify its properties and uses. Let’s take a closer look at each of these elements.
Tantalum is a transition element found in Group 5 of the periodic table. It has an atomic weight of 180.9 and an atomic number of 73. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d3. This element is used in a variety of applications, including electronics, aerospace, and medical equipment. It is also used in the production of jewelry and other decorative items.
Iridium is a transition element found in Group 6 of the periodic table. It has an atomic weight of 192.2 and an atomic number of 77. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d7. This element is known for its strength and durability, making it a popular choice for industrial applications. It is also used in the production of electronic components and catalytic converters.
Astatine (At)
Astatine is a halogen found in Group 7 of the periodic table. It has an atomic weight of 210.0 and an atomic number of 85. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p5. This element is highly radioactive and is used in the medical field for diagnosing and treating certain types of cancers. It is also used in research and development for a variety of scientific purposes.
Now that you know the element associated with the electron configuration 1s2 2s2 2p6 3s2 3p6, you can better understand the properties and uses of each element. Tantalum is used in a variety of industrial applications, iridium is known for its strength and durability, and astatine is used in medical and research applications. Understanding the electron configuration of elements can be a helpful tool in identifying their properties and uses.
What element is 1s2 2s2 2p6 3s2 3p6 4s2 3d1 3d1 3d1?
The electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d1 3d1 3d1 is the atomic structure of an element with atomic number 73, commonly known as tantalum. It belongs to the transition element group and has an atomic weight of 180.9.
Tantalum is one of the rare earth metals that are essential to modern technology, and is used in the manufacturing of capacitors, surgical equipment, and superalloys. It is highly resistant to corrosion and has a high melting point, making it an ideal material for a wide range of applications.
Tantalum: Properties and Uses
Tantalum has several unique properties that make it a valuable material for industrial and medical uses. Its high melting point of 3,457 degrees Celsius makes it ideal for use in high-temperature applications. It is also highly resistant to corrosion, meaning it can be used in a variety of environments without fear of corrosion.
Tantalum is also extremely ductile, meaning it can be formed into thin wires and sheets, making it ideal for use in electrical components and other small parts. It is also a good conductor of electricity, making it useful for wiring and circuit boards. In addition, it is highly biocompatible, making it a great material for surgical tools and implants.
Uses of Tantalum
Tantalum is used in a wide range of industrial and medical applications. It is a common component in capacitors, as its high melting point makes it ideal for this purpose. It is also used in the manufacturing of superalloys, which are used in the aerospace and automotive industries.
In addition, tantalum is used in the medical field for surgical tools and implants. Its biocompatibility makes it an ideal material for use in medical instruments, and its corrosion resistance makes it a great material for implants and prosthetics.
Tantalum vs Iridium
Tantalum and iridium are both transition elements with similar electron configurations. Iridium has an atomic number of 77 and an atomic weight of 192.2. While both elements have similar properties, iridium has a higher melting point of 4,453 degrees Celsius, making it ideal for even higher temperature applications.
In addition, iridium is denser than tantalum, making it more suitable for use in high-density applications. It is also more corrosion-resistant than tantalum, making it a better choice for use in environments where corrosion is a concern.
Overall, tantalum and iridium are both valuable transition elements with similar properties. Tantalum is a better choice for lower temperature applications and is more ductile than iridium, while iridium is better suited for higher temperature applications and is more corrosion-resistant. Both elements have their place in modern technology and will continue to be used for a variety of applications.



Leave a Comment