A Pyridine Dearomatization Approach to the Matrine-type Lupin Alkaloids with Jeff Kerkovius
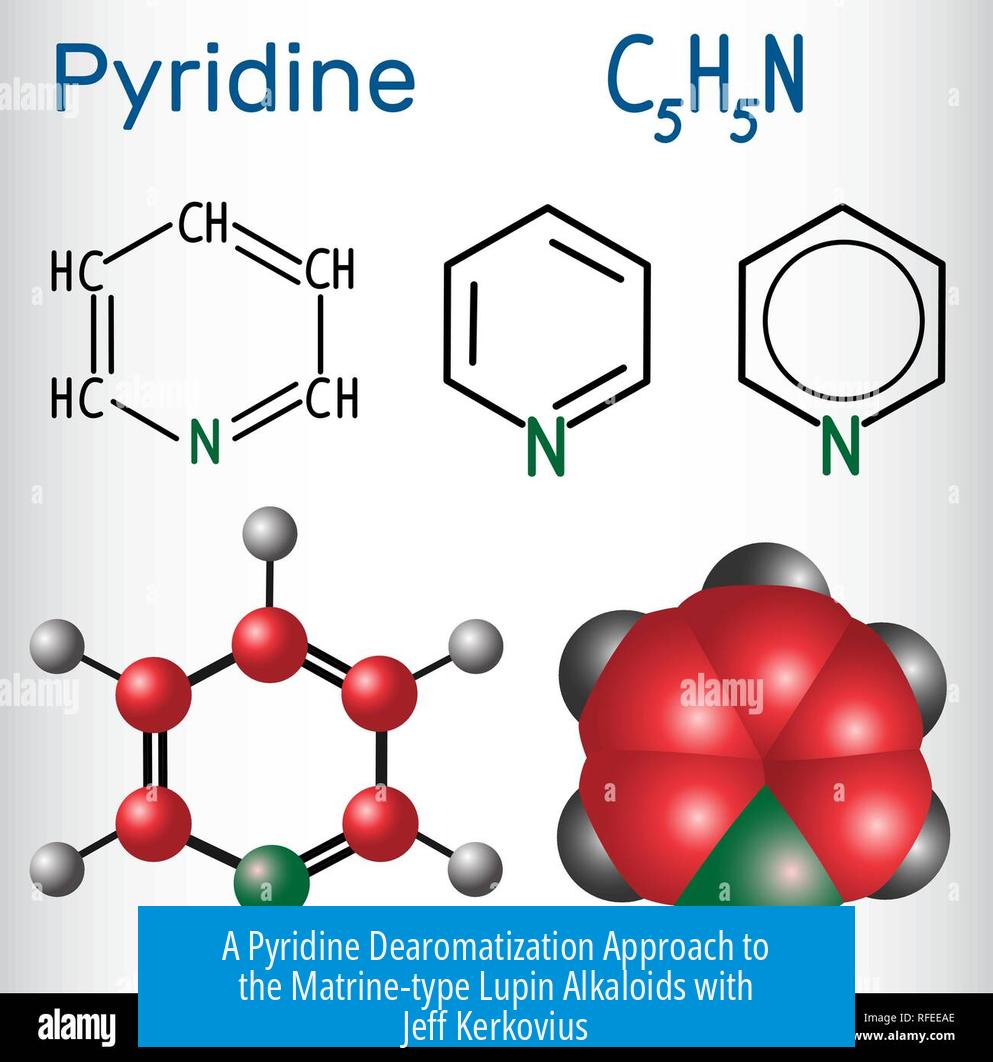
Jeff Kerkovius from the Reisman group at Caltech developed a pyridine dearomatization method to efficiently synthesize matrine-type lupin alkaloids. This work highlights a novel strategy using simple reaction conditions to build complex alkaloid structures.
Background and Significance
Matrine-type lupin alkaloids are a class of bioactive natural products found in lupin plants. Their complex frameworks pose challenges for synthetic chemists. Kerkovius’ approach bypasses traditional multi-step syntheses by directly transforming pyridine, a readily available aromatic compound, through dearomatization—a process that converts the aromatic ring into a more reactive, non-aromatic intermediate.
Methodology Overview
- Pyridine is subjected to mild reaction conditions that induce dearomatization.
- This reaction facilitates the formation of key complex intermediates central to matrine-type alkaloid frameworks.
- The approach maintains stereochemical control, as suggested by charge and stereochemical assignments (+ and –) on the molecules discussed.
- Overall, the synthesis occurs under simple, accessible conditions while achieving complex molecular architectures.
Impact and Reception
This synthetic strategy was published in the Journal of the American Chemical Society (2022, Vol.144, pp.15938–15943), reflecting its significance. The work excited the organic chemistry community due to its elegance and efficiency. The methodology bridges traditional synthetic organic chemistry and innovative reaction design, which surprised some by its placement outside purely synthetic journals.
On the Synthesis Workshop YouTube channel, Kerkovius discusses this project in detail, enabling broader access and understanding of the experimental setup and results.
Additional Insights and Discussion Points
- The notation of “+” and “–” on molecules likely indicates stereochemical orientation or ionic charge states critical to the reaction’s outcome.
- Informal remarks about “boofing” or questioning whether these compounds are “drugs” highlight intrigue about biological properties and lab vernacular but do not affect the chemistry.
Key Takeaways
- A pyridine dearomatization method enables efficient construction of matrine-type lupin alkaloids.
- The synthesis uses straightforward reaction conditions to yield complex natural product architectures.
- The approach achieves stereochemical control relevant for bioactive alkaloid structures.
- Published in JACS (2022), illustrating its importance and novelty in organic synthesis.
- Accessible resources like the synthesis workshop video provide practical insights into the experimental method.
A Pyridine Dearomatization Approach to the Matrine-type Lupin Alkaloids with Jeff Kerkovius – New on Synthesis Workshop!
Jeff Kerkovius unveils a clever pyridine dearomatization strategy to craft matrine-type lupin alkaloids, blending elegance and simplicity in synthetic design. This innovative approach, recently featured on Synthesis Workshop, showcases how straightforward conditions yield intricate molecular architectures, a feat that excites both seasoned chemists and aspiring synthetic enthusiasts.
Ever wondered how complex natural products like matrine-type lupin alkaloids come to life in the lab? Jeff Kerkovius, from the Reisman group at Caltech, spills the secrets in a detailed YouTube episode, walking viewers through his method that balances chemical finesse with practical ease. Let’s explore why this approach stands out and what it means for organic synthesis.
What’s the big deal about pyridine dearomatization?
Pyridine rings are notoriously stable because of their aromaticity — they don’t give up their flat, delocalized electrons easily. Dearomatization means intentionally disrupting this aromatic stability to transform the pyridine into a more reactive, three-dimensional intermediate. This shift opens doors to constructing complex molecules that are otherwise tough to access.
In Jeff Kerkovius’s work, the dearomatized pyridine serves as a springboard to build matrine-type lupin alkaloids—a class of natural molecules derived from plants in the lupin family. These alkaloids catch the eye due to their potential biological activity and structural complexity. Yet, making them synthetically has long posed a challenge.
Simple conditions, complex results

What’s truly striking about this paper (J. Am. Chem. Soc., 2022, 144, 15938-15943) is how unpretentious the reaction setup is. Jeff and his team manage to tame a complicated synthetic target using very accessible reagents and mild conditions. It’s a refreshing reminder that you don’t always need a kitchen full of exotic chemicals to synthesize intricate natural products.
“This was a sweet paper.” — An enthusiastic nod from the community appreciating the blend of simplicity and sophistication.
Such straightforward methodologies lure more researchers to explore similar dearomatization tactics for other alkaloid families. It sparks curiosity: Could simplicity be the next trend in complex molecule synthesis?
Crossing boundaries — more than just organic synthesis
Interestingly, the project blurs lines between traditional organic synthesis and other chemistry subfields. Some noted surprise it wasn’t published in a central nervous system (CNS) related journal, hinting at its relevance not just synthetically but potentially pharmacologically.
What’s more, Jeff’s talk on Synthesis Workshop has spurred questions buzzing in the comments section. People are intrigued by the molecules’ stereochemical notations—what do those plus and minus signs mean on some structures? They point to chirality and stereochemical configurations, critical features that control biological activities in these alkaloids.
Is it drugs? Should you “boof it”?
Among playful banter, some wonder if they’re watching alkaloid-based drug synthesis or some “mad scientist” concocting something illicit. The answer: No, it’s genuine synthetic organic chemistry aimed at understanding molecules with medicinal potential, not street chemistry. The “boof it” comment is tongue-in-cheek—highlighting how chemists riff on jargon while discussing their craft.
Why does this matter to you?
If you’re dabbling in organic synthesis or fascinated by natural product chemistry, Jeff’s pyridine dearomatization tactic offers a fresh lens. It invites you to rethink how aromatic stability can be temporarily undone to carve complex skeletons methodically.
Plus, the publicly available YouTube episode (https://youtu.be/fWvfpqnW_5U) lets you peek into the minds of top researchers. It’s less a dry lecture, more a lively workshop, perfect to inspire your next lab project or challenge preconceived notions about reaction complexity.
Practical takeaways for synthetic chemists
- Leverage dearomatization: Use it as a tactical tool to unlock new molecular shapes.
- Embrace mild conditions: Don’t overcomplicate your syntheses; sometimes simple reagents and gentle setups do the heavy lifting.
- Study stereochemistry carefully: Recognize that + and – signs matter for product function, guiding your chiral control strategies.
- Expand your horizons: This work isn’t just about making molecules; it bridges to biological relevance, offering potential pathways to drug discovery.
Final thoughts: A synthesis story worth exploring
Jeff Kerkovius’s pyridine dearomatization approach is more than clever chemistry. It’s a storytelling tool in molecular innovation, showing how breaking aromaticity can build natural product frameworks traditionally deemed complex. The approach debunks myths that sophistication requires complicated chemistry.
For students and professionals alike, this method offers a blueprint: respect the fundamentals, manipulate key motifs with precision, and share your findings openly—which is exactly what Jeff does via the Synthesis Workshop platform.
So, next time you face a stubborn aromatic ring limiting your synthetic creativity, ask: What if I dearomatize it? What new pathways might that unlock?
Check out the episode, dive into the JACS paper, and let this synthesis adventure spark new ideas in your lab or study—because in the world of chemistry, even the most stubborn ring can take a new shape with the right touch.
What is the main strategy used in Jeff Kerkovius’s synthesis of matrine-type lupin alkaloids?
The approach relies on a pyridine dearomatization step. This step enables the construction of complex lupin alkaloid frameworks using relatively simple reaction conditions.
Why is the reaction considered straightforward despite producing complex molecules?
The method uses mild and accessible conditions that avoid complicated setups. This simplicity contrasts with the structural complexity of the resulting alkaloids.
Where can I watch the full presentation on this synthesis approach?
The entire episode featuring Jeff Kerkovius is available on YouTube at synthesis-workshop.com or directly via the link: https://youtu.be/fWvfpqnW_5U.
How does this work bridge different areas of chemistry?
It blends organic synthesis with innovative transformations uncommon in typical synthetic routes. The work’s placement outside purely organic journals reflects this interdisciplinary nature.
What do the plus and minus signs mean on the molecules discussed?
These symbols usually refer to stereochemical configurations or charge states in the chemical structures shown. They help clarify the precise form of the synthesized alkaloids.



Leave a Comment