Alcohols with SOCl2 and TsCl
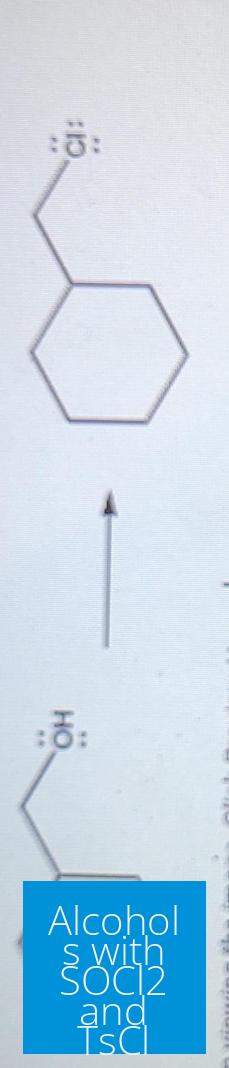
When converting alcohols to better leaving groups for substitution reactions, both thionyl chloride (SOCl2) and tosyl chloride (TsCl) serve key roles. SOCl2 transforms alcohols into alkyl chlorides with inversion of stereochemistry. TsCl converts alcohols into tosylates, improving leaving ability without altering stereochemistry.
1. Why Convert Alcohols into Better Leaving Groups?
Alcohols have a hydroxide (OH) group that acts as a leaving group in substitution reactions.
- Hydroxide ion (OH−) is a strong base and thus a poor leaving group, which hinders substitution steps.
- For efficient nucleophilic substitution, a leaving group must be weakly basic and stable once departed.
- Converting alcohols to better leaving groups allows smooth substitution to other functional groups.
Two common routes to improve leaving groups from alcohols are:
- Conversion to sulfonate esters using TsCl (tosyl chloride) or MsCl (mesyl chloride).
- Conversion to alkyl chlorides using SOCl2.
2. Conversion of Alcohols with Thionyl Chloride (SOCl2)
Reaction Overview
SOCl2 transforms primary and secondary alcohols into alkyl chlorides. This process produces hydrochloric acid (HCl) and sulfur dioxide (SO2) as gaseous byproducts.
Reaction Equation (Simplified):
ROH + SOCl2 → RCl + SO2 + HCl
Stereochemistry and Mechanism
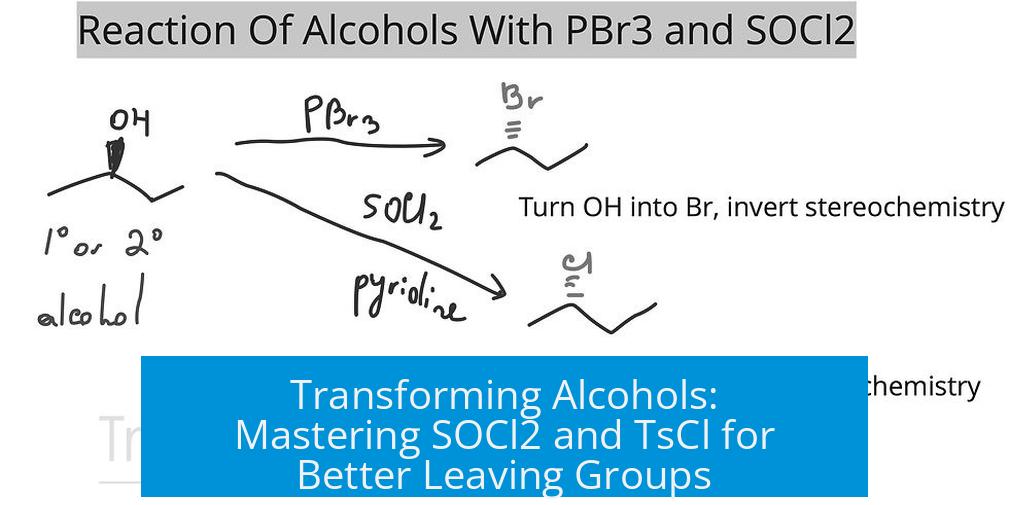
- The reaction proceeds via an SN2 mechanism involving backside attack by chloride ion.
- This leads to inversion of configuration at the chiral carbon center.
- SOCl2 first reacts with the alcohol oxygen, forming an intermediate and releasing a chloride ion.
- The chloride then attacks the carbon bearing the leaving group, breaking the C–O bond and inverting stereochemistry.
Solvent Effects
While the reaction is generally associated with inversion of stereochemistry, the stereochemical outcome can vary depending on the solvent used. Some solvents allow partial retention, leading to mixtures of stereoisomers.
Additional Reactions
- SOCl2 also converts carboxylic acids into acid chlorides by a mechanism involving nucleophilic addition of chloride to the carbonyl carbon.
- The conversion of acid hydroxyl groups to acid chlorides facilitates further nucleophilic acyl substitution reactions.
3. Conversion of Alcohols with Tosyl Chloride (TsCl)
Reaction Overview
Tosyl chloride (TsCl) converts alcohols into tosylates (alkyl sulfonate esters). These leaving groups are superior to hydroxide but differ mechanistically from SOCl2 reactions.
Key Points:
- The sulfonate ester formed is a good leaving group, facilitating subsequent substitution or elimination.
- Tosylation does not alter stereochemistry, meaning retention occurs at the chiral center.
- This property is vital when preserving stereochemistry during multi-step synthesis.
Mechanism
- The alcohol oxygen attacks the sulfur atom of TsCl, displacing chloride ion.
- This forms the tosylate ester while leaving the stereoconfiguration unchanged.
Reactivity Compared to Alkyl Chlorides
Tosylates generally react slower with nucleophiles than alkyl chlorides due to their lower electrophilicity. This makes them useful intermediates where controlled reaction rates are desirable.
4. Detailed Mechanisms
SOCl2 Mechanism – Stepwise
- The alcohol oxygen nucleophilically attacks sulfur in SOCl2, displacing one chloride to form an alkyl chlorosulfite intermediate.
- A chloride ion generated in this reaction attacks the carbon bearing the newly formed leaving group, performing backside attack.
- C–O bond breaks; inversion of stereochemistry occurs; the alkyl chloride is released.
- Decomposition of the chlorosulfite intermediate yields SO2 gas and HCl.
Tosylation Mechanism
- Alcohol oxygen attacks sulfur in TsCl, displacing chloride ion.
- Tosylate ester forms with the oxygen bonded to the sulfonyl group.
- No formation of carbocation or inversion; stereochemistry remains intact.
5. Laboratory and Safety Considerations
SOCl2 Handling
- Thionyl chloride is volatile with a strong pungent odor described as sickly sweet.
- Exposure to moisture or breathing vapors can release corrosive gases HCl and SO2.
- Must be handled in a well-ventilated fume hood with proper protective equipment.
- Accidental spills may cause rapid release of sulfur dioxide and hydrochloric acid gases, posing risks to health and requiring evacuation.
Tosyl Chloride Handling
- Tosyl chloride is less volatile and easier to handle than SOCl2.
- Still requires gloves and eye protection due to irritant properties.
6. Comparative Summary of SOCl2 and TsCl in Alcohol Conversion
| Feature | SOCl2 | TsCl |
|---|---|---|
| Resulting Product | Alkyl chloride (R–Cl) | Tosylate ester (R–OTs) |
| Leaving Group Strength | Good leaving group (Cl−) | Very good leaving group (tosylate ion) |
| Reaction Mechanism | SN2 with inversion of configuration | Formation of sulfonate ester with retention of configuration |
| Stereochemical Outcome | Generally inversion, solvent dependent | No change (retention) |
| Byproducts | SO2 and HCl gases | HCl (from base scavenger) |
| Typical Usage | Prepare alkyl chlorides for further transformations; convert acids to acyl chlorides | Activate alcohols for substitution or elimination without stereochemical change |
| Reaction Rate | Fast; SN2 | Slower than chlorides |
7. Specialized Applications and Additional Notes
- Allylic and benzylic alcohols may be converted to chlorides using tosylation followed by displacement with chloride ion.
- Reactions using TsCl can be combined with other reagents such as LiCl and triethylamine to facilitate chloride displacement.
- The choice of method depends on desired stereochemical outcome and reaction conditions.
- Tosylates can serve as intermediates where substitution conditions require milder environments or stereochemical retention.
- SOCl2 offers a direct route to alkyl halides but demands strict handling precautions.
Key Takeaways
- Alcohols are poor leaving groups due to the strong basicity of hydroxide.
- SOCl2 converts alcohols to alkyl chlorides via an SN2 mechanism, causing inversion of stereochemistry.
- Tosyl chloride (TsCl) converts alcohols to tosylates without changing stereochemistry (retention).
- SOCl2 also converts carboxylic acids to acid chlorides, facilitating acyl substitution reactions.
- The choice of reagent depends on stereochemical and synthetic needs.
- SOCl2 reactions produce hazardous gases; handle under strict safety conditions.
- Tosylates serve as versatile intermediates in organic synthesis with good leaving group ability.
Mastering Alcohols with SOCl2 and TsCl: Why Chemists Love Turning Hydroxyls into Leaving Stars
So, what’s the big deal with alcohols, SOCl2, and TsCl? Simply put: alcohols are a bit stubborn when it comes to replacing that pesky hydroxyl group (–OH) in synthesis reactions. Hydroxide (HO^–) isn’t a star leaving group – it’s a strong base and a rather poor one at that. Fortunately, with reagents like thionyl chloride (SOCl2) and tosyl chloride (TsCl), chemists can swap out that hard-to-leave hydroxyl for something much better. Let’s dive into this fascinating world where chemistry meets clever conversion.
The Challenge: Hydroxide’s Bad Reputation as a Leaving Group
Imagine you want to take an alcohol and turn it into something else via substitution. The problem? Hydroxide ion is a lousy leaving group. Why? Because it’s a strong base, and strong bases dislike leaving. They cling stubbornly, making reactions slow or impossible. That’s like trying to get a guest who’s had one too many drinks to leave the party—good luck!
The solution is to turn the alcohol into a better leaving group first. This is where *sulfonate esters* and *alkyl chlorides* come into play.
Transforming Alcohols into Better Leaving Groups: TsCl vs. SOCl2
To improve the leaving group ability of the hydroxyl, chemists have two tricks: convert the alcohol into a sulfonate ester using tosyl chloride (TsCl) or mesyl chloride (MsCl), or convert it into an alkyl chloride using thionyl chloride (SOCl2). Both achieve the goal but behave differently in mechanism and stereochemistry.
- Tosylates and Mesylates: React alcohols to create sulfonate esters (like tosylates). These leave readily in substitution steps and are generally stereochemically neutral—they keep the same configuration.
- Alkyl Chlorides via SOCl2: Turn alcohols into alkyl chlorides, which are versatile and reactive intermediates for further transformations.
Both methods improve the “leaving group skill set” of the alcohol but do so with unique quirks we’ll explore below.
How SOCl2 Works: Reaction Mechanism and Stereochemistry in Action
Adding SOCl2 to an alcohol produces an alkyl chloride, hydrochloric acid (HCl), and sulfur dioxide (SO2) as byproducts. These gaseous side products bubble away, making the reaction neat and less messy to purify.
The reaction proceeds through an SN2 mechanism—a swift backside attack by a chloride ion on the carbon bonded to the oxygen. The first step involves the alcohol oxygen attacking the sulfur atom on SOCl2. This activates the –OH group, transforming it into a better leaving partner, often called a chlorosulfite intermediate.
Then chloride ion performs the nucleophilic attack on the carbon bearing the leaving group, pushing out the activated group and flipping the stereochemistry. This inversion of configuration is akin to turning a glove inside out. The mechanism’s backside attack ensures this inversion.
| Step | Description | Byproducts |
|---|---|---|
| 1 | Alcohol oxygen attacks SOCl2 sulfur → chlorosulfite intermediate | None (yet) |
| 2 | Chloride ion does SN2 attack on carbon → alkyl chloride formed | HCl and SO2 gases release |
*Note:* While introductory students are told this always results in full inversion of stereochemistry, in reality, there’s a solvent-dependent dance going on—sometimes you get inversion, sometimes retention, or a mix. Chemistry loves throwing curveballs!
Tosyl Chloride: Sulfonate Esters That Don’t Mess with Your Stereo
TsCl turns alcohols into tosylates. These sulfonate esters serve as excellent leaving groups because sulfonates are stable and weakly basic. Unlike SOCl2, the reaction with TsCl does not invert stereochemistry. Your chiral centers stay put—making TsCl an elegant choice when stereochemical integrity is crucial.
The reaction mechanism is more straightforward here: the alcohol attacks the sulfur on TsCl, displacing chloride, forming the tosylate ester. A base deprotonates the intermediate, yielding the sulfonate. No nucleophilic attack on the carbon attached to the oxygen occurs at this stage, so no inversion happens.
Why Could You Choose SOCl2 Over TsCl, or Vice Versa?
- Speed and Reactivity: Alkyl chlorides formed via SOCl2 are usually more reactive than tosylates. If you want fast substitution later, chlorides might be better.
- Stereochemistry: SOCl2 reacts with inversion; TsCl keeps configuration steady. This is critical in making chiral drugs or stereoselective intermediates.
- Reaction Conditions: SOCl2 reactions often require refluxing and emit nasty gases; TsCl reactions are typically mild and cleaner.
- Substrate Specificity: Primary and secondary alcohols work well with both. Tertiary alcohols don’t cooperate with SOCl2 but might go via other routes.
SOCl2 and Carboxylic Acids: A Bonus Conversion
Here’s the magic trick: SOCl2 also transforms carboxylic acids (R–COOH) into acid chlorides (R–COCl). This conversion frees up the carboxyl group to react in nucleophilic acyl substitution without the unhelpful hydroxyl group getting in the way.
The mechanism here involves a [1,2]-addition where chloride attacks the carbonyl carbon, followed by elimination of SO2 and HCl. This leaves behind the reactive acid chloride, a prized intermediate in organic synthesis.
Chemistry Lab Tales: The Not-So-Fun Side of SOCl2
Thionyl chloride is famous in labs—mostly for its NOSE. It has a pungent, nauseating, sickly-sweet smell that lingers like an uninvited guest at a party. One anecdote recalls a student accidentally dropping 5 mL of SOCl2 into a rotovap bath, unleashing a cloud of SO2 and HCl that evacuated the whole lab for half an hour.
This stuff needs handling with extreme care—like phosgene-level caution. Always work in a fume hood, wear gloves, and have your safety gear on. You do *not* want this cloud inside your lungs.
Leaving Group Strength Versus Nucleophilicity: What’s the Difference?
Chloride ions are interesting creatures. They make for decent leaving groups but aren’t super-powered bases. This balance lets them leave when pushed but not so readily that they cause chaos.
In comparison, tosylates are excellent leaving groups too but tend to be less reactive, leading to slower but more controlled reactions. This choice boils down to the needs of your synthesis road trip.
Transforming Allylic and Benzylic Alcohols: A Special Case
Here’s a fun chemistry hack: allylic alcohols like geraniol can be converted into their corresponding chlorides (e.g., geranyl chloride) using methanesulfonyl chloride with lithium chloride (LiCl) and triethylamine (NEt3). This protocol leverages the ease with which these substrates undergo substitution due to resonance stabilization and neighboring group effects.
In such cases, tosylation followed by nucleophilic substitution with chloride is the winning combo, especially when working with electron-rich benzylic or allylic alcohols that favor SN1 mechanisms.
Wrapping It Up: Practical Tips for Chemists!
- Need inversion of stereochemistry? Go with SOCl2, but watch your solvents for side surprises.
- Want to keep your chiral center’s configuration? TsCl likes to keep things steady without flipping.
- Hate noxious fumes? TsCl is your gentle friend; SOCl2 is more like the bad boy that leaves a lasting impression.
- Working with allylic or benzylic alcohols? Consider tosylates and nucleophilic chloride substitution for a smooth ride.
- Turn carboxylic acids into acyl chlorides quickly: SOCl2 makes this fast and efficient, but safety first!
“Treat thionyl chloride like your worst ex—don’t get too close!”
Ultimately, the choice between SOCl2 and TsCl depends on what puzzle piece you need: a swift nucleophilic substitution with flip-flop configuration or a patient, stereochemically-retentive partner. Understanding these reagents’ quirks helps chemists sculpt molecules with surgical precision.
Alcohols may start with a humble –OH, but with SOCl2 and TsCl in hand, we give them VIP passes to the grand ball of organic transformations.
1. Why are alcohols converted using SOCl2 or TsCl?
Alcohols have poor leaving groups due to the hydroxide ion. SOCl2 or TsCl converts the –OH into better leaving groups like alkyl chlorides or sulfonate esters, making substitution reactions easier.
2. How does SOCl2 change the stereochemistry of alcohols?
SOCl2 reacts via an S_N2 mechanism, which leads to inversion of configuration at the carbon center. This backside attack flips the stereochemistry compared to the original alcohol.
3. Does TsCl affect stereochemistry like SOCl2?
No, TsCl converts alcohols into tosylates without altering the stereochemistry. The reaction proceeds with retention, unlike the inversion seen with SOCl2 reactions.
4. What are the byproducts formed when alcohols react with SOCl2?
When SOCl2 converts an alcohol, hydrochloric acid (HCl) and sulfur dioxide (SO2) are formed as byproducts. Both gases typically escape from the reaction mixture.
5. Can SOCl2 also react with carboxylic acids?
Yes, SOCl2 converts carboxylic acids into acid chlorides. This helps facilitate further reactions by replacing the hydroxyl group with a more reactive chlorine atom.
6. How does solvent choice affect the SOCl2 reaction?
The extent of inversion versus retention during the SOCl2 reaction depends on the solvent used. Different solvents can lead to mixed stereochemical outcomes, not always pure inversion.


Leave a Comment