Atoms are the essential building blocks of the universe. Every atom consists of three particles: protons, neutrons, and electrons. These particles determine the identity of an element, and the number of protons in an atom dictates its atomic number. A neutral atom also has an equal number of electrons, while the number of neutrons can vary, resulting in different isotopes of an element. With so many different elements and isotopes, it can be difficult to keep track of all the different combinations.
This article is dedicated to answering the question: what elements have 150 neutrons? We’ll explore the different isotopes of each element and look at the atomic number, mass number, and number of protons, neutrons, and electrons in each. We’ll also look at two different ways that isotopes are written and the mass of each particle. Finally, we’ll break down the atomic structures of the first six elements and discuss a few other related questions.
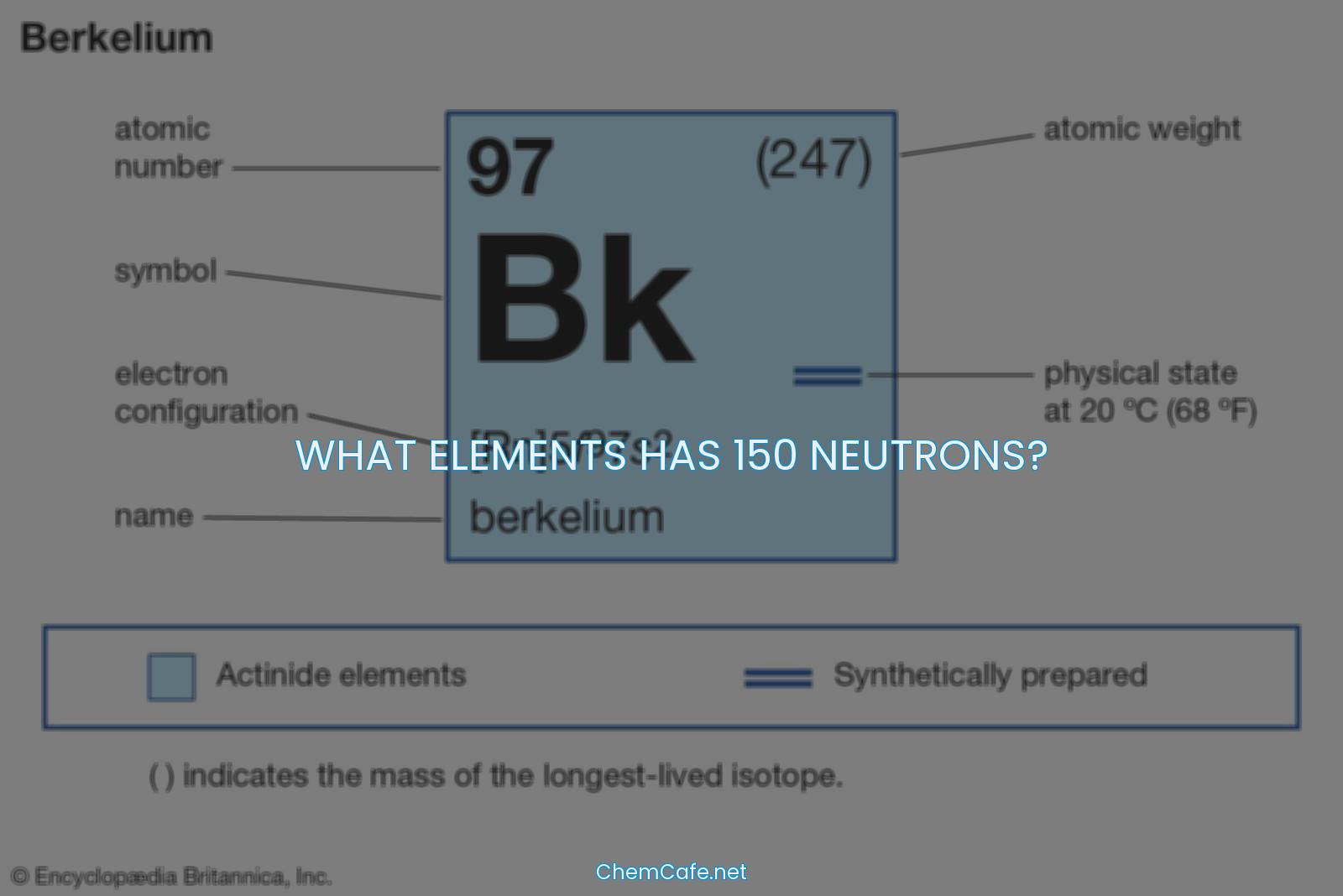
Atoms are composed of protons, neutrons, and electrons, and these particles dictate the identity of an element. Protons have a positive charge and are found in the nucleus of the atom, while electrons have a negative charge and orbit around the nucleus. Neutrons have no charge and are also found in the nucleus. Every atom of an element has the same number of protons – this number is the atomic number and it helps identify the element. Of course, since neutral atoms have to have one electron for every proton, an element’s atomic number also tells you how many electrons are in a neutral atom of that element.
Isotopes are identified by their mass, which is the total number of protons and neutrons. There are two ways that isotopes are generally written. They both use the mass of the atom where mass = (number of protons) + (number of neutrons). One atomic mass unit is the mass of a proton, or about (1.67 times 10^{-27}) kilograms, which is an extremely small mass. A neutron has just a tiny bit more mass than a proton, but its mass is often assumed to be one atomic mass unit as well. Because electrons have virtually no mass, just about all the mass of an atom is in its protons and neutrons.
Now that we have a basic understanding of atoms, let’s take a look at the different elements with 150 neutrons. We’ll start with a table of the first six elements and their atomic numbers, mass numbers, and the number of protons, neutrons, and electrons in each. We’ll then move on to the isotopes of each element and the two different ways in which isotopes are written. Finally, we’ll answer a few related questions to help you better understand the structure of elements.
What elements has 150 neutrons?
Atoms are made of protons, neutrons, and electrons. The number of protons in an atom determines what element it is. This number is called the atomic number (Z) and is the same for all atoms of an element. The number of neutrons in an atom can vary, however, and atoms with the same number of protons but different numbers of neutrons are called isotopes.
Atoms of the same element can have different numbers of neutrons, and therefore different mass numbers (A). The mass number is the total number of protons and neutrons in an atom. The number of neutrons in an atom can be determined by subtracting the atomic number from the mass number.
Table (PageIndex{1}): Atoms of the First Six Elements
| Element | Protons | Neutrons | Electrons | Atomic Number (Z) | Mass Number (A) |
|---|---|---|---|---|---|
| Hydrogen | 1 | 0 | 1 | 1 | 1 |
| Helium | 2 | 2 | 2 | 2 | 4 |
| Lithium | 3 | 4 | 3 | 3 | 7 |
| Beryllium | 4 | 5 | 4 | 4 | 9 |
| Boron | 5 | 6 | 5 | 5 | 11 |
| Carbon | 6 | 6 | 6 | 6 | 12 |
Of course, since neutral atoms have to have one electron for every proton, an element’s atomic number also tells you how many electrons are in a neutral atom of that element. Isotopes are identified by their mass, which is the total number of protons and neutrons. There are two ways that isotopes are generally written. They both use the mass of the atom where mass = (number of protons) + (number of neutrons).
One atomic mass unit is the mass of a proton, or about (1.67 times 10^{-27}) kilograms, which is an extremely small mass. A neutron has just a tiny bit more mass than a proton, but its mass is often assumed to be one atomic mass unit as well. Because electrons have virtually no mass, just about all the mass of an atom is in its protons and neutrons.
So, how many neutrons does an element with 150 neutrons have? The answer depends on the element. The atomic number of the element will determine how many protons it has, and subtracting the atomic number from the mass number will tell you how many neutrons it has. For example, if the element has an atomic number of 82, then it has 82 protons and 150 – 82 = 68 neutrons. If the element has an atomic number of 92, on the other hand, then it has 92 protons and 150 – 92 = 58 neutrons.
The element with 150 neutrons can be any of a number of different elements, depending on the atomic number. Some examples of elements with 150 neutrons include barium (56 protons and 94 neutrons), cesium (55 protons and 95 neutrons), and lead (82 protons and 68 neutrons). All of these elements have 150 neutrons, but have different numbers of protons.
What atom has 94 electrons?
Atoms are made up of protons, neutrons, and electrons. Electrons are the smallest particles in an atom and are responsible for the chemical properties of an atom. The number of electrons in an atom is determined by its atomic number, which is the number of protons in the nucleus of the atom.
Atomic number 94 is plutonium. Plutonium has 94 protons and 94 electrons, with 146 neutrons. Plutonium is a radioactive element of the actinide series and is found in trace amounts in nature. It is produced in nuclear reactors and is used in nuclear weapons and energy production.
The Electron Configuration of Plutonium
The electron configuration of plutonium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 5f7 6d1.
The first two electrons go to the 1s orbital, which is the lowest energy orbital. The next two electrons go to the 2s orbital, the next highest energy orbital. After the 2s orbital is filled, the next six electrons occupy the 2p orbital, and then the 3s orbital is filled. The 3d and 4s orbitals are next and then the 4p, 4d, and 5s orbitals are filled. The 5p orbital is then filled, followed by the 4f, 5d, 6s, and 6p orbitals. The last electron is placed in the 6d orbital.
The Properties of Plutonium
Plutonium is a silver-gray metal with a melting point of 641°C (1,186°F). It is highly radioactive and has a half-life of 24,000 years. Plutonium is a toxic element, and contact with it can cause severe health problems.
Plutonium is used in nuclear weapons and nuclear energy production. It is also used in medical applications and in aerospace technology.
Atomic number 94 is plutonium, and it has 94 electrons and 146 neutrons. The electron configuration of plutonium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 5f7 6d1. Plutonium is a silver-gray metal with a melting point of 641°C (1,186°F). It is highly radioactive and has a half-life of 24,000 years. Plutonium is used in nuclear weapons and nuclear energy production, as well as in medical and aerospace applications.
What metal has 125 neutrons?
Atoms with different numbers of neutrons are known as isotopes, and they can be found in different elements. For example, hydrogen has three isotopes – protium, deuterium, and tritium – each with different numbers of neutrons. So, which metal has 125 neutrons?
The answer is uranium, which is a heavy metal with an atomic number of 92 and a mass number of 238. Uranium is the heaviest naturally occurring element on Earth and is found in trace amounts in soil and rocks. It is also the primary fuel used in nuclear reactors and bombs.
Uranium has 92 protons and 125 neutrons, which gives it a mass number of 238. This means that each atom of uranium contains 92 protons and 125 neutrons, for a total of 238 particles. Uranium is an unstable element, meaning that it can undergo radioactive decay, releasing energy in the form of radiation.
The Difference between Atomic Number and Mass Number
When talking about atoms, the atomic number (Z) is the number of protons in the nucleus and the mass number (A) is the total number of protons and neutrons in the nucleus. So, for uranium, the atomic number is 92, and the mass number is 238.
The atomic number of an element is always the same, while the mass number can vary due to the presence of different isotopes. For example, hydrogen has three isotopes – protium (1 proton, 0 neutrons), deuterium (1 proton, 1 neutron), and tritium (1 proton, 2 neutrons).
What is an Isotope?
An isotope is an atom of a particular element that has a different number of neutrons than other atoms of the same element. Isotopes of a given element have the same number of protons, but different numbers of neutrons. This means that each isotope of a given element has a different mass number.
For example, uranium-238 is an isotope of uranium that has 92 protons and 146 neutrons, while uranium-235 is another isotope of uranium with 92 protons and 143 neutrons. These two isotopes of uranium have different mass numbers, but the same atomic number.
Uses of Uranium
Uranium is a very important element in the nuclear industry. It is used as fuel in nuclear reactors and bombs, and it is also used in medical imaging and radiotherapy. Uranium is also used in production of nuclear weapons, nuclear submarines, and nuclear power plants.
Uranium is also used in some industries, such as ceramics, glassmaking, and jewelry. It is also used in some medical applications, such as radiopharmaceuticals and radiation therapy.
So, what metal has 125 neutrons? The answer is uranium – a heavy metal with an atomic number of 92 and a mass number of 238. Uranium has 92 protons and 125 neutrons, which gives it a mass number of 238. Uranium is an unstable element, meaning that it can undergo radioactive decay, and is used in many industries, including the nuclear industry, ceramics, glassmaking, and jewelry.
What element has 122 neutrons?
Atoms are made up of protons, neutrons, and electrons. The number of protons and electrons in an atom determines what element it is. The number of neutrons, however, can vary. This means that atoms of the same element can have different numbers of neutrons, and are called isotopes.
The mass of an atom is the sum of the number of protons and neutrons it has. This number is called the mass number or atomic mass. Isotopes are identified by their mass number, which tells us how many protons and neutrons the atom has.
So, the element with 122 neutrons is the isotope of an element with an atomic mass of 122. This could be any element, but the most common elements with an atomic mass of 122 are tin, tellurium, and lead.
Tin-122
Tin-122 is an isotope of tin, with 50 protons and 72 neutrons. It is the most common isotope of tin, and accounts for nearly half of all natural tin. Tin-122 is an unstable isotope, and it undergoes beta decay to form the stable isotope tin-124.
Tellurium-122
Tellurium-122 is an isotope of tellurium, with 52 protons and 70 neutrons. It is the most stable isotope of tellurium, and accounts for nearly a third of all natural tellurium. Tellurium-122 is also radioactive, but it has a much longer half-life than tin-122, at over 2 million years.
Lead-122
Lead-122 is an isotope of lead, with 82 protons and 40 neutrons. It is the second most common isotope of lead, after lead-204. Lead-122 is a stable isotope, and it does not undergo any type of natural decay.
Why Are There Different Isotopes?
Atoms of the same element can have different numbers of neutrons, which means that they have different mass numbers. This is because the number of neutrons in an atom is determined by the process of nuclear fusion. During this process, lighter elements are fused together to form heavier elements, and the number of neutrons in the atom can vary depending on the elements that are fused together.
The element with 122 neutrons is any element with an atomic mass of 122, such as tin, tellurium, and lead. The reason why there are different isotopes of an element is because the number of neutrons in an atom is determined by nuclear fusion.
What element has 146 neutrons?
Uranium, symbolized as (ce{^{238}_{92}U}), is an element with 146 neutrons. This element is part of the actinide series, and is the heaviest element known to exist in large quantities on Earth. It is used as a fuel for nuclear reactors and in the manufacture of nuclear weapons.
Uranium is a metallic, silver-gray element with an atomic number of 92. This means it has 92 protons and 92 electrons in its atomic structure. Its atomic mass is 238, which includes the 146 neutrons. There are two main isotopes of uranium, with masses of 235 and 238.
Uranium Chemistry and Metallurgy
Uranium-235 contains 143 neutrons in addition to its 92 protons, while uranium-238 contains 146 neutrons. Despite the different number of neutrons, the two isotopes of uranium behave in the same way when it comes to chemical reactions.
Uranium is used in a wide variety of applications, including metallurgy and nuclear energy. In metallurgy, uranium is used to make alloys, which are materials made from two or more metals. Alloys are often stronger and more durable than the metals used to make them.
Natural Decay Series of Uranium-238
Uranium-238 is a naturally decaying element. Its decay is part of a series of natural processes called the decay series. This process begins with the original element, which then decays into a series of new elements.
Uranium-238 decays into a series of elements including protactinium, thorium, radium, radon, and lead. Each element in the decay series has a different number of neutrons. As uranium-238 decays, the number of neutrons in the nucleus slowly decreases.
Uranium is used in a variety of applications, including nuclear energy. In the nuclear energy industry, uranium is used as a fuel for nuclear reactors. This fuel is a highly efficient source of energy, and is used to generate electricity.
Uranium is also used in the manufacture of nuclear weapons. This element is essential for the production of nuclear bombs, as it is the primary element used in the explosive core.
Uranium is also used in the medical field, in the form of radioisotopes. These radioisotopes are used for diagnosis and treatment of various medical conditions, including cancer.
Conclusion
Uranium is an element with an atomic number of 92 and an atomic mass of 238. This element has two main isotopes, uranium-235 and uranium-238. Uranium-238 has 146 neutrons in its nucleus and is used in a variety of applications, including nuclear energy, metallurgy, and the manufacture of nuclear weapons. Radioisotopes of uranium are also used in the medical field for the diagnosis and treatment of various medical conditions.
What element has 144 neutrons?
Most elements have multiple isotopes, which are atoms with the same number of protons, but different numbers of neutrons. One of the elements with an isotope containing 144 neutrons is plutonium. The two main isotopes of plutonium are plutonium-238 and plutonium-239. Plutonium-238 has 94 protons and 144 neutrons, resulting in a mass number of 238, while plutonium-239 has 94 protons and 145 neutrons, resulting in a mass number of 239.
What is Plutonium?
Plutonium is a radioactive metal that is solid at room temperature and has a bright silver appearance. It is also one of the heaviest elements, twenty times heavier than water. Plutonium is both chemically and radiotoxic, so it must be handled with extreme care.
Uranium is another element with isotopes that have 144 neutrons. Uranium-235 has 92 protons and 143 neutrons, while uranium-238 has 92 protons and 146 neutrons. Both isotopes of uranium have the same chemical behavior, even though the mass numbers are different.
The mass number of an element includes both protons and neutrons, but not electrons. This is because protons and neutrons are both part of an atom’s nucleus and have much greater mass than electrons. Since electrons are outside of the nucleus, they have much less of an effect on an atom’s mass number.
Uses of Plutonium and Uranium
Plutonium and uranium are both used in nuclear power plants. Plutonium is used to fuel nuclear reactors, while uranium is used in both nuclear reactors and nuclear weapons. Both elements are also used in the medical field, as both are radioactive and can be used to treat certain diseases.
Plutonium-238 and uranium-235 are two elements that have isotopes with 144 neutrons. Plutonium is a radioactive metal that is solid at room temperature and is very heavy. It is used in nuclear power plants and has medical applications. Uranium is also used in nuclear power plants and nuclear weapons. Both elements have mass numbers that include both protons and neutrons, but not electrons.


Leave a Comment