What Is the Chemical Formula of Drinking Water?
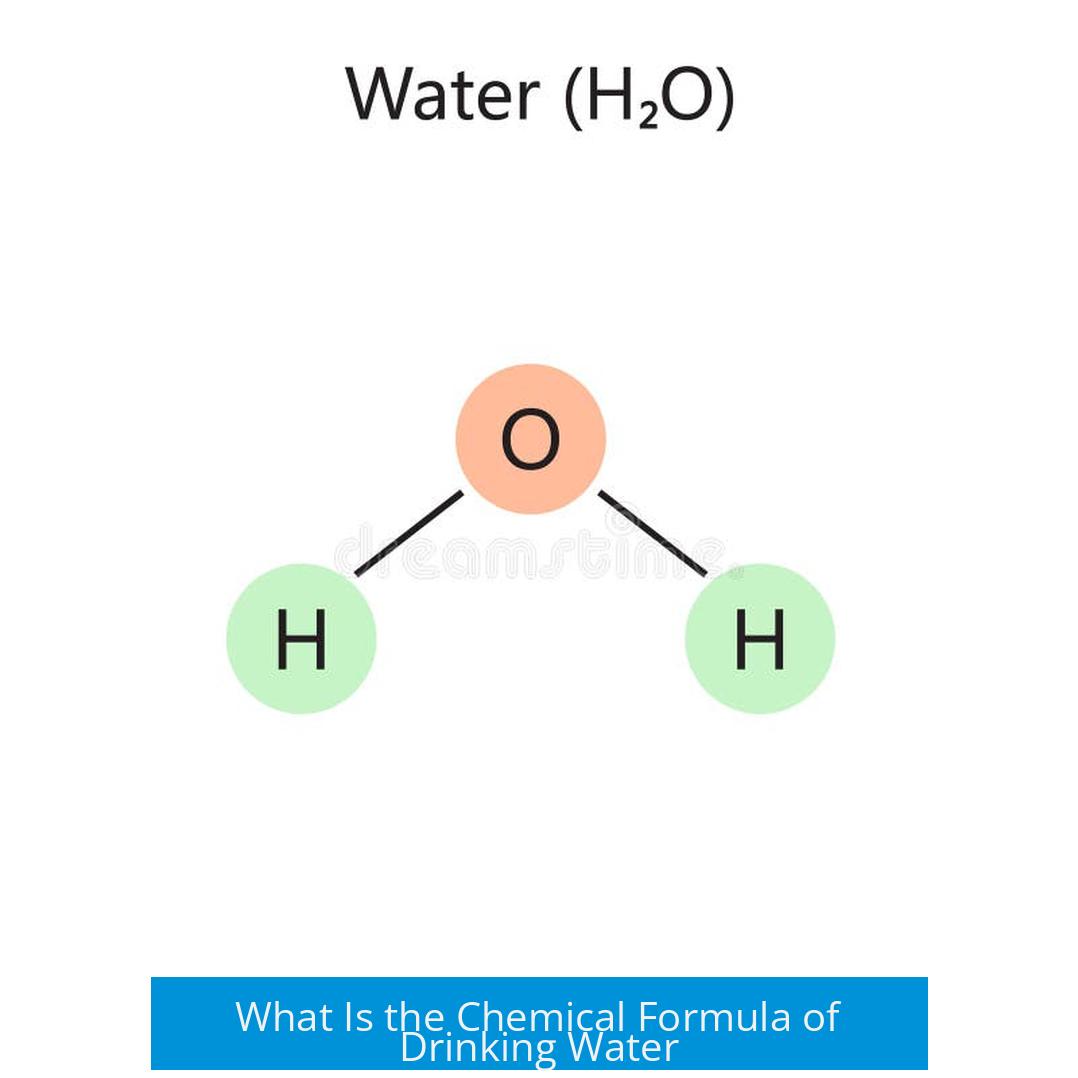
The chemical formula of drinking water cannot be represented by a single formula, as drinking water is a mixture of many chemicals with varying compositions. Its main component is pure water, which has the chemical formula H2O, but the overall mixture changes depending on source, treatment, and impurities.
Chemical Formula of Pure Water
Water’s true chemical formula is H2O. Each molecule consists of two hydrogen atoms bonded to one oxygen atom. This formula applies to all pure water, regardless of source.
Drinking Water as a Chemical Mixture
Drinking water is not a single chemical but a mixture primarily made up of water molecules. It contains dissolved minerals, ions, and trace elements that vary widely:
- Calcium (Ca2+) and magnesium (Mg2+) ions often lead to “hard water.”
- Low mineral content produces “soft water.”
- Trace impurities may include sodium, iron, fluoride, or even heavy metals like lead in polluted areas.
Since mixtures do not have fixed chemical formulas, drinking water as a whole cannot be expressed by a unique formula. Instead, each dissolved component has its own chemical identity.
Variability and Reporting of Drinking Water Composition

The exact makeup depends on the water source, the treatment process, and distribution system. Local water suppliers provide water quality reports listing these components with their concentrations. These reports help define acceptable impurity limits and monitor safety.
Understanding Solutions in Chemistry
Dissolving substances in water is a physical change, not a chemical one. Therefore, solutions do not receive a new chemical formula. Chemists specify water’s formula as H2O separately from the solutes’ chemical formulas and give their concentration in the solution.
Summary of Key Points
- Drinking water is a mixture; it has no single chemical formula.
- Pure water’s chemical formula is H2O.
- Impurities vary by location and treatment, affecting water qualities.
- Hard and soft water differ based on mineral ion concentration.
- Chemists report individual solutes separately, not as a combined formula.
What is the chemical formula of drinking water?
Drinking water has no single chemical formula because it is a mixture of many chemicals. It mostly contains water (H2O) but also various dissolved minerals and impurities.
Why can’t drinking water have a single chemical formula like pure water?
Mixtures do not have chemical formulas. Drinking water varies by source and treatment, so its composition changes. This variability prevents assigning one formula.
What is the chemical formula of pure water, the main part of drinking water?
Pure water’s formula is H2O, representing two hydrogen atoms bonded to one oxygen atom. This is the true chemical formula for water itself.
How do minerals in water affect its classification?
Water with high levels of minerals like calcium or magnesium is called hard water. Low mineral content water is soft water. These minerals affect taste and usage.
How do water quality reports relate to drinking water’s chemical makeup?
Local water suppliers publish reports listing chemicals and impurities in drinking water. These help monitor safety and show what chemicals are present besides H2O.




Leave a Comment