Are you trying to figure out how to determine the number of protons, neutrons and electrons in an atom or ion? You’ve come to the right place! In this post, we’ll be going through the steps to help you find the number of protons, neutrons, and electrons in an atom or ion. We’ll first start by discussing what each of the components in the nuclide notation means. Then, we’ll provide two examples together.
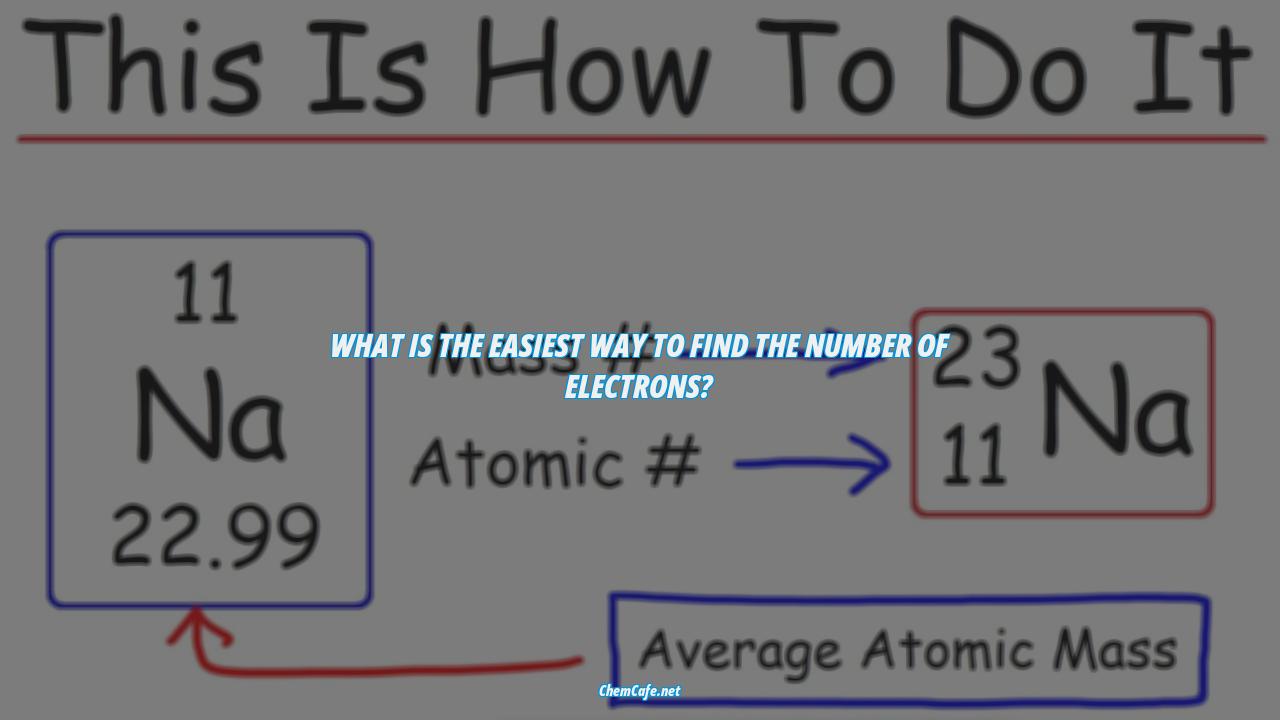
The nuclide notation is a shorthand used to represent the number of each type of particle in an atom or ion. It consists of three parts: the letter(s) in the middle represents the symbol of the element, the number on the bottom left corner is the atomic number which tells you the number of protons, and the number on the upper left corner is the mass number, which is equal to the number of protons and neutrons added together. Lastly, the charge is on the upper right corner. If there isn’t any number or signs, then it means that atom has no charge and is neutral.
We’ll then provide some simple rules to help you determine the number of protons, neutrons, and electrons in an atom or ion. The number of protons is equal to the atomic number, the number of neutrons is equal to the mass number minus the atomic number, and the number of electrons is equal to the atomic number minus the charge.
We’ll provide two examples to help you understand how to implement the rules. We’ll use the Periodic Table of Elements to help us find the atomic number and atomic weight of our elements. We’ll use the example of krypton and explain how many protons, electrons, and neutrons are in an atom of krypton. We’ll also provide some information about the electron configuration and how to write it.
Finally, we’ll provide some special cases for ions and explain the reason for the formation of those ions. We’ll explain that if you need to write the full electron configuration for an anion, then you are just adding additional electrons and the configuration is simply continued.
With the information provided in this post, you’ll be able to find the number of protons, neutrons, and electrons in an atom or ion. You’ll also be able to learn how to write the electron configuration and learn some special cases for ions. Good luck!
What is the easiest way to find the number of electrons?
The number of protons, neutrons, and electrons in an atom or ion can be determined easily with a few simple steps. Knowing the nuclide notation and the associated rules can help in understanding the structure of an atom. In this post, we’ll discuss the nuclide notation and the rules to find the number of protons, neutrons, and electrons in an atom. We’ll also go through two examples together to make the concept more clear.
The Nuclide Notation
The nuclide notation is the way in which the components of an atom are written. It consists of the element symbol in the middle, the atomic number on the bottom left corner, the mass number on the upper left corner and the charge on the upper right corner. The atomic number is the number of protons in an atom, while the mass number is the total number of protons and neutrons added together. Lastly, the charge tells you the number of electrons in an atom.
Rules to Finding Number of Protons, Neutrons, and Electrons
To find the number of protons, neutrons and electrons in an atom, you just need to remember three simple rules:
- Number of protons = atomic number
- Number of neutrons = mass number – atomic number
- Number of electrons = atomic number – charge
Examples
Let’s apply the rules to some examples. How many protons, electrons and neutrons are in an atom of krypton, carbon, oxygen, neon, silver, gold, etc.?
To find the number of protons, electrons and neutrons in an atom, just follow these easy steps:
- Step 1 – Gather Information: The first thing you will need to do is find the information about your element. Go to the Periodic Table of Elements and click on your element. If it makes things easier, you can select your element from an alphabetical listing. Use the Table of Elements to find your element’s atomic number and atomic weight.
- Step 2 – The Number of Protons is: The atomic number is the number of protons in an atom of an element. In our example, krypton’s atomic number is 36. This tells us that an atom of krypton has 36 protons in its nucleus.
Order of Fill
The order in which electrons are placed into the orbitals is based on the order of their energy. This is referred to as the Aufbau principle. The lowest energy orbitals fill first. Just like the quantum numbers themselves this order was determined by calculation and is summarized by the following chart:
:max_bytes(150000):strip_icc()/Order-of-Fill-5c4f7cab46e0fb0001f3a1a0.jpg)
Alternatively, you can just use the periodic table to find the number of protons, electrons and neutrons in an atom.
How to Write an Electron Configuration
The symbols used for writing the electron configuration start with the shell number (n) followed by the type of orbital and finally the superscript indicates how many electrons are in the orbital. For example:
Looking at the periodic table, you can see that Oxygen has 8 electrons. Based on the order of fill above, these 8 electrons would fill in the following order 1s, 2s and then 2p. So Oxygen’s electron configuration would be O 1s22s22p4.
Special Cases
Configurations of ions present a special case of electron configuration and also demonstrate the reason for the formation of those ions in the first place. If you need to write the full electron configuration for an anion, then you are just adding additional electrons and the configuration is simply continued.
For a neutral atom, the number of protons is exactly equal to the number of electrons. So the number of electrons is the same as the atomic number. However, it is possible to remove electrons and not change the identity of an element. These are called ions. The charge on the ion tells you the number of electrons. If the charge is positive, subtract that number from the atomic number to get the number of electrons. You have more protons. If the charge is negative, add the amount of charge to the atomic number to get the number of electrons. You have more electrons.
Atoms
Complete the Table:
| isotopes | # protons | # neutrons | # electrons |
|---|---|---|---|
| 85Rb | 37 | 48 | 37 |
| 119Sn4+ | 50 | 69 | 46 |
| 13C4- | 6 | 7 | 10 |
| 235U | 92 | 143 | 92 |
| 24Na+ | 11 | 13 | 11 |
| 223Fr+ | 87 | 136 | 87 |
In conclusion, finding the number of protons, electrons and neutrons in an atom is easy once you understand the nuclide notation and the associated rules. All you need to remember is the atomic number, mass number and the charge of the atom to determine the number of protons, electrons and neutrons. Using the periodic table can also help in finding the number of each component in an atom.
We hope that this post helped in understanding the concept of finding the number of protons, neutrons and electrons in an atom. For more information on atomic structure, check out the links below.
Atomic Structure Links
- What is an Atom?
- What is Atomic Structure?
- Quantum Numbers in Atomic Structure
- What is an Ion?
- What is an Isotope?
- Chemical Demonstration Videos
How do you find the total number of electrons?
Atoms are composed of protons, neutrons, and electrons, and the number of electrons in a given atom can determine its chemical properties and behavior. To calculate the total number of electrons in a molecule or ion, one must first determine the electron configurations for each element in the molecule, then add up all the electrons. This article will discuss the basics of electron configurations and the process for determining the total number of electrons in a molecule or ion.
The Electron Configurations of Atoms
An atom’s electron configuration is a representation of the number of electrons in each sublevel in each energy level of the ground-state atom. To determine the electron configuration of a particular atom, start at the nucleus and add electrons one by one until the number of electrons equals the number of protons in the nucleus. Each added electron is assigned to the lowest-energy sublevel available. The first sublevel filled will be the 1s sublevel, then the 2s sublevel, the 2p sublevel, the 3s, 3p, 4s, 3d, and so on.
This order is difficult to remember and often hard to determine from energy-level diagrams such as Figure 5.8. A more convenient way to remember the order is to use Figure 5.9. The principal energy levels are listed in columns, starting at the left with the 1s level. To use this figure, read along the diagonal lines in the direction of the arrow. The order is summarized under the diagram.
How to Calculate the Total Number of Electrons
The process for calculating the total number of electrons in a molecule or ion is relatively straightforward. Here is the step-by-step process:
Step 1: Determine the number of atoms of each element in the molecule or ion.
Step 2: For each element, calculate the number of electrons by multiplying the number of atoms by the atomic number of the element.
Step 3: Add up all the products to calculate the number of electrons.
Step 4: Subtract the charge value from the number of electrons obtained in Step 3 if the ion has a positive charge. Add the charge value to the number of electrons (Step 3) if the ion has a negative charge. Skip this step if the molecule has a neutral charge.
Example Calculations
To illustrate the process, let’s look at two examples. The first example is the ion KNO3, and the second example is the ion SO42-.
Example 1 (KNO3): The number of atoms of each element in KNO3 is 1 K, 1 N, and 3 O. To calculate the number of electrons, multiply the number of atoms by the atomic number of each element: (19 x 1) + (7 x 1) + (8 x 3) = 50.
Example 2 (SO42-): The number of atoms of each element in SO42- is 1 S and 4 O. To calculate the number of electrons, multiply the number of atoms by the atomic number of each element: (16 x 1) + (8 x 4) = 48.
Step 5: Subtract the charge value from the number of electrons obtained in Step 3 if the ion has a positive charge. Add the charge value to the number of electrons (Step 3) if the ion has a negative charge. Skip this step if the molecule has a neutral charge.
In our examples, only SO42- is a charged ion; it has the negative charge 2. Add this value to the total from Step 3 to determine the total number of electrons in the molecule: 48 +2 = 50.
Order of Fill
The order in which electrons are placed into the orbitals is based on the order of their energy. This is referred to as the Aufbau principle. The lowest energy orbitals fill first. Just like the quantum numbers themselves this order was determined by calculation and is summarized by the following chart:
How to Write an Electron Configuration
The symbols used for writing the electron configuration start with the shell number (n) followed by the type of orbital and finally the superscript indicates how many electrons are in the orbital.
For example:
Looking at the periodic table, you can see that Oxygen has 8 electrons. Based on the order of fill above, these 8 electrons would fill in the following order 1s, 2s and then 2p. So Oxygen’s electron configuration would be O 1s22s22p4.
Special Cases
Configurations of ions present a special case of electron configuration and also demonstrate the reason for the formation of those ions in the first place. If you need to write the full electron configuration for an anion, then you are just adding additional electrons and the configuration is simply continued.
Electron configurations are an important concept in chemistry, as they are used to determine the valency of an element, predict the properties of a group of elements, and interpret atomic spectra. To calculate the total number of electrons in a molecule or ion, one must first determine the electron configurations for each element in the molecule, then add up all the electrons. The process is relatively straightforward but may involve special cases, such as ions, which require additional steps.
How do you find the number of electrons?
Finding the number of electrons in an atom or ion is an important part of understanding how atoms and molecules interact with each other. Knowing the number of electrons can help us better understand the structure of atoms and how they interact with one another. In this post, we will discuss how to determine the number of protons, neutrons, and electrons in an atom or ion. By the end of this post, you will have a better understanding of the nuclide notation and how to find the number of protons, neutrons, and electrons.
The Nuclide Notation
Before we start, let’s discuss what the nuclide notation is. This notation is usually written in the form of A Z X, where A is the mass number, Z is the atomic number, and X is the charge. The mass number is the combined number of protons and neutrons in the nucleus, while the atomic number is the number of protons in the nucleus. Lastly, the charge is the number of protons minus the number of electrons.
Rules to Finding Number of Protons, Neutrons, and Electrons
Now that you understand the nuclide notation, let’s discuss the rules for finding the number of protons, neutrons, and electrons.
Number of protons = atomic number
Number of neutrons = mass number – atomic number
Number of electrons = atomic number – charge
Examples
Now let’s put our knowledge to the test with some examples. For a neutral atom, the number of protons is exactly equal to the number of electrons. So the number of electrons is the same as the atomic number. However, it is possible to remove electrons and not change the identity of an element. These are called ions. The charge on the ion tells you the number of electrons. If the charge is positive, subtract that number from the atomic number to get the number of electrons. You have more protons. If the charge is negative, add the amount of charge to the atomic number to get the number of electrons. You have more electrons.
Complete the Table
Let’s take a look at some isotopes and complete the table below.
| Isotopes | # Protons | # Neutrons | # Electrons |
|---|---|---|---|
| 85Rb | 37 | 48 | 37 |
| 119Sn4+ | 50 | 69 | 46 |
| 13C4- | 6 | 7 | 10 |
| 235U | 92 | 143 | 92 |
| 22Na+ | 11 | 13 | 11 |
| 87Fr+ | 13 | 68 | 68 |
Atomic Structure Links
If you’re looking for more information on atomic structure, here are some useful links and resources:
We hope this post has given you a better understanding of how to find the number of protons, neutrons, and electrons in an atom or ion. By following the rules of the nuclide notation and using the examples given, you should now have a better idea of how to go about finding this information. If you have any questions, feel free to reach out. Happy learning!
How do you determine the number of electrons?
Atoms are composed of protons, neutrons, and electrons. These three components make up the nucleus and the electrons are arranged in different shells around the nucleus. Knowing the number of protons, neutrons, and electrons in an atom or ion is essential to understanding their behavior and properties. In this article, we’ll discuss how to determine the number of protons, neutrons, and electrons in an atom or ion.
The Nuclide Notation
The nuclide notation is used to represent an atom or ion. The letter(s) in the middle is the symbol of the element. The number on the bottom left corner is the atomic number, which tells us the number of protons. The number on the upper left corner is the mass number, which is equal to the sum of the neutrons and protons. Lastly, the charge is on the upper right corner. If there isn’t any number or signs, then it means that atom has no charge and is neutral.
Rules to Finding Number of Protons, Neutrons, and Electrons
To determine the number of protons, neutrons, and electrons in an atom or ion, you can use the following rules:
- Number of protons = atomic number
- Number of neutrons = mass number – atomic number
- Number of electrons = atomic number – charge
Examples
Let’s apply the rules to some examples. Let’s say we have an atom with an atomic number of 28. Since it is a neutral atom, the number of protons and electrons is equal to the atomic number, which is 28. To find the number of neutrons, we need to subtract the atomic number from the mass number. So, in this case, the number of neutrons is 28 – 28 = 0.
Now, let’s consider an ion with an atomic number of 13 and a charge of +4. To find the number of protons, we can use the atomic number, which is 13. The number of electrons is equal to the atomic number minus the charge, which is 13 – 4 = 9. The number of neutrons is equal to the mass number minus the atomic number, which is 13 – 13 = 0.
What is a Proton Number and an Ion?
Proton Number: The proton number, also called the atomic number, of an element is the number of protons in one atom of that element. The proton number can be found on the periodic table of elements.
Ion: An ion is a charged atom. In a neutral atom, the number of protons and electrons are equal. In a positively charged ion, also called a cation, the number of protons is greater than the number of electrons.
The Electron Configurations of Atoms
The electron configuration of an atom shows the number of electrons in each sublevel in each energy level of the ground-state atom. To determine the electron configuration of a particular atom, start at the nucleus and add electrons one by one until the number of electrons equals the number of protons in the nucleus. Each added electron is assigned to the lowest-energy sublevel available. The first sublevel filled will be the 1s sublevel, then the 2s sublevel, the 2p sublevel, the 3s, 3p, 4s, 3d, and so on. This order is difficult to remember and often hard to determine from energy-level diagrams.
A more convenient way to remember the order is to use a diagram known as Figure 5.9. The principal energy levels are listed in columns, starting at the left with the 1s level. To use this figure, read along the diagonal lines in the direction of the arrow. The order is summarized under the diagram.
Ions and Atoms
Ions are atoms with a charge due to the loss or gain of electrons. When an atom has lost or gained electrons, the number of electrons is no longer equal to the number of protons. To determine the number of electrons in an ion, you need to take into account the charge of the ion.
If the charge is positive, subtract that number from the atomic number to get the number of electrons. You have more protons. If the charge is negative, add the amount of charge to the atomic number to get the number of electrons. You have more electrons.
For example, a neutral atom of hydrogen has an atomic number of 1 and one proton and one electron. An ion of sodium has an atomic number of 11 and a charge of +1. The number of electrons in the sodium ion is 11 – 1 = 10.
Determining the number of protons, neutrons, and electrons in an atom or ion is essential for understanding the behavior and properties of atoms and ions. By using the nuclide notation and following the rules outlined in this article, you can easily calculate the number of protons, neutrons, and electrons.
By understanding the structure of atoms and ions, scientists can gain insight into the behavior of matter and the different properties of materials. This knowledge can be used to create new materials with specific properties and to design technologies that can improve our lives.
How do you count electrons in a molecule?
The number of electrons in a molecule can be determined by counting the electrons of each element and then adding them up. This can be done using the atomic number of each element, which is the number of protons in the nucleus. The number of electrons in the molecule is equal to the sum of the atomic numbers of all the elements in the molecule.
Steps to calculate the number of electrons in a molecule
The calculation of the number of electrons in a molecule involves several steps. Here is an overview of these steps:
Step 1: Calculate the number of atoms of each element
The first step is to determine the number of atoms of each element in the molecule. This can be done by looking at the chemical formula of the molecule. The chemical formula of a molecule shows the number of atoms of each element present in the molecule.
For example, the chemical formula of potassium nitrate (KNO3) shows that there are 1 atom of potassium (K), 1 atom of nitrogen (N), and 3 atoms of oxygen (O) present in the molecule.
Step 2: Find the atomic number of each element
The next step is to find the atomic number of each element. This can be done by looking up the element in the periodic table. The atomic number of an element is the number of protons in the nucleus. It is also equal to the number of electrons in the neutral atom.
For example, the atomic number of potassium (K) is 19, the atomic number of nitrogen (N) is 7, and the atomic number of oxygen (O) is 8.
Step 3: Multiply the atomic number of each element by the number of atoms of that element
The third step is to multiply the atomic number of each element by the number of atoms of that element in the molecule. This will give the total number of electrons of that element in the molecule.
For example, in KNO3, there is 1 atom of potassium (K) and its atomic number is 19. The number of electrons of potassium in the molecule is 1 x 19 = 19. Similarly, there are 1 atom of nitrogen (N) and its atomic number is 7. The number of electrons of nitrogen in the molecule is 1 x 7 = 7. There are 3 atoms of oxygen (O) and its atomic number is 8. The number of electrons of oxygen in the molecule is 3 x 8 = 24.
Step 4: Add up the number of electrons of all the elements
The fourth step is to add up the number of electrons of all the elements in the molecule. This will give the total number of electrons in the molecule.
For example, in KNO3, the total number of electrons is 19 + 7 + 24 = 50.
Step 5: Subtract or add the charge value if the molecule has a charged ion
The fifth step is to subtract or add the charge value from the total number of electrons in the molecule if the molecule has a charged ion. If the ion has a positive charge, the charge value should be subtracted from the total number of electrons. If the ion has a negative charge, the charge value should be added to the total number of electrons.
For example, sulfur dioxide (SO2) has a negative charge of 2. The total number of electrons in the molecule is 48 (16 x 1 + 8 x 4). The charge value of 2 should be added to the total number of electrons in the molecule. So, the total number of electrons in the molecule is 50 (48 + 2).
What are Electron Configurations?
Electron configurations describe how electrons are distributed in atomic orbitals. This can be written in a standard notation, which indicates the energy level and type of orbital, followed by the number of electrons in the orbital written in superscript.
For example, the electron configuration of carbon (atomic number: 6) is 1s22s22p2. This indicates that there are 2 electrons in the 1s orbital, 2 electrons in the 2s orbital, and 2 electrons in the 2p orbital.
Rules to Follow While Writing the Electronic Configuration of Elements
There are three rules that must be followed while writing the electronic configuration of elements. These rules are the Aufbau Principle, Pauli’s Exclusion Principle, and Hund’s Rule of Maximum Multiplicity.
The Aufbau Principle
The Aufbau Principle states that electrons must completely fill the atomic orbitals of a given energy level before occupying an orbital associated with a higher energy level. This means that the lowest energy orbitals fill first.
Pauli’s Exclusion Principle
Pauli’s Exclusion Principle states that no two electrons can have equal values for all four quantum numbers. Consequently, each subshell of an orbital can accommodate a maximum of 2 electrons and both these electrons MUST have opposite spins.
Hund’s Rule of Maximum Multiplicity
Hund’s Rule of Maximum Multiplicity states that all the subshells in an orbital must be singly occupied before any subshell is doubly occupied. Furthermore, the spin of all the electrons in the singly occupied subshells must be the same (in order to maximize the overall spin).
The number of electrons in a molecule can be determined by counting the electrons of each element and then adding them up. This can be done using the atomic number of each element, which is the number of protons in the nucleus. The number of electrons in the molecule is equal to the sum of the atomic numbers of all the elements in the molecule. The calculation of the number of electrons in a molecule involves several steps, including calculating the number of atoms of each element, finding the atomic number of each element, multiplying the atomic number of each element by the number of atoms of that element, and adding up the number of electrons of all the elements. Additionally, the electron configuration of elements is written using a standard notation, and three rules must be followed while writing the electronic configuration of elements.
How do you find the number of electrons without a charge?
Atoms are composed of protons, neutrons, and electrons. The protons and neutrons make up the nucleus, while the electrons are located around the nucleus in an electron cloud. The number of protons and electrons in an atom is the same, meaning that atoms are neutral and have no overall charge. However, when an atom gains or loses electrons, it creates an ion. The number of electrons in an ion can be determined by its charge.
Atomic Structure
Atoms consist of protons, neutrons, and electrons. The number of protons in an atom is equal to its atomic number, which is listed on the periodic table. The number of neutrons is equal to the mass number minus the atomic number. The number of electrons is equal to the number of protons, meaning that a neutral atom has no overall charge. However, when an atom gains or loses electrons, it becomes an ion.
Ions and Atoms
Ions can be either cations (positively charged atoms) or anions (negatively charged atoms). To determine if an ion is a cation or an anion by looking at the symbol, check the charge of the ion written on the top right of the element. If the number is positive, the ion is a cation. If the number is negative, the ion is an anion.
Counting Protons and Electrons in Atomic Ions
The number of protons in an atom is always equal to its atomic number. For example, the atomic number of potassium (K) is 19. Therefore, the number of protons is 19. To find the number of electrons in an ion, we can subtract the charge of the ion from the atomic number. For example, if we have an ion with the symbol K18+, the number of electrons is 18 (19 – 1).
How to Find the Number of Protons, Neutrons, and Electrons
The number of protons, neutrons, and electrons in an atom can be determined from the nuclide notation. The letter(s) in the middle is the symbol of the element. The number on the bottom left corner is the atomic number, which tells you the number of protons. The number on the upper left corner is the mass number, which is equal to the neutrons and protons added together. Lastly, the charge is on the upper right corner. If there isn’t any number or signs, then it means that atom has no charge and is neutral.
The rules to finding the number of protons, neutrons, and electrons are as follows:
- Number of protons = atomic number
- Number of neutrons = mass number – atomic number
- Number of electrons = atomic number – charge
Let’s practice counting protons and electrons in atomic ions with the next two examples.
Example 1
Complete the following table by filling in the blanks as necessary for the symbol, type of ion, number of protons, or number of electrons for an atomic ion.
| Symbol | Type of Ion | Number of protons | Number of electrons |
|---|---|---|---|
| K18+ | Cation | 19 | 1 |
Step 1: Find the number of protons in an atomic ion. The number of protons is equal to the atomic number of the element. The atomic number of potassium (K) is 19. Therefore, the number of protons is 19.
Step 2: Find the number of electrons in an atomic ion. In a neutral atom, the number of electrons is equal to the number of protons. For an ion, charged positively or negatively, the number of electrons is the atomic number (the number of protons) minus the charge of the ion. In this example, the charge is 18, so the number of electrons is 19 – 18 = 1.
Step 3: Determine if an ion is a cation or an anion. In this example, the number of electrons is 1, which is fewer than the number of protons (19). Therefore, this ion is a cation.
Example 2
Complete the following table by filling in the blanks as necessary for the symbol, type of ion, number of protons, or number of electrons for an atomic ion.
| Symbol | Type of Ion | Number of protons | Number of electrons |
|---|---|---|---|
| Na11- | Anion | 11 | 12 |
Step 1: Find the number of protons in an atomic ion. The number of protons is equal to the atomic number of the element. The atomic number of sodium (Na) is 11. Therefore, the number of protons is 11.
Step 2: Find the number of electrons in an atomic ion. In a neutral atom, the number of electrons is equal to the number of protons. For an ion, charged positively or negatively, the number of electrons is the atomic number (the number of protons) minus the charge of the ion. In this example, the charge is 11, so the number of electrons is 11 + 11 = 12.
Step 3: Determine if an ion is a cation or an anion. In this example, the number of electrons is 12, which is more than the number of protons (11). Therefore, this ion is an anion.
In conclusion, the number of electrons in an atom can be determined without a charge by looking at the nuclide notation. The number of protons is equal to the atomic number, and the number of electrons is equal to the atomic number minus the charge. By comparing the number of electrons and the number of protons, you can determine whether an ion is a cation or an anion.
How do you find electrons per shell?
Atoms are composed of three basic components: protons, neutrons, and electrons. Electrons are the smallest particles and are found in the outermost layer of an atom, known as shells or energy levels. The number of electrons in each shell determines an element’s chemical behavior. This article will discuss how to find electrons per shell, the importance of electron configurations, and provide examples of electron configurations.
How to Find Electrons Per Shell
The shells are orbital paths that are followed by electrons around the nucleus. Like everything in chemistry, electrons like to follow the path of least resistance. This means that electrons will usually fill up the shells of an atom from the inside out. Starting at the lowest energy level and working its way out.
Each shell has a maximum amount of electrons it can hold. For example, shell 1n can hold 2 electrons, shell 2n can hold 8 electrons, and shell 3n can hold 18 electrons. The rule to calculate the number of electrons that each shell can hold is 2n2. E.g. the first shell is 2(1)2 which gives you 2 electrons. In the diagram above the energy levels are depicted as the rings around the nucleus of the atom. The outermost layer of electrons is known as its valence shell. The valence shell electrons determine an element’s affinity to bond. When valence shells are full this usually is a good indicator that the electron is stable. The magic number when it comes to valence shells is the number eight.
Predicting an Electron Arrangement
The electron arrangement of an atom can be found out from its atomic number. E.g., the atomic number of sodium is 11 which means that the sodium atom has 11 protons and 11 electrons where:2 electrons occupy the first shell, 8 electrons occupy the second shell and1 electron occupies the third shell.This electron arrangement can be written as 2, 8, 1. The electronic arrangement of the first 18 elements is listed below:
Name of Element | Symbol | Atomic Number | KLMN | Valency
Hydrogen | H | 1 | ––– | 1
Helium | He | 2 | ––– | 2
Lithium | Li | 3 | 2––– | 1
Beryllium | Be | 4 | 2–– | 2
Boron | B | 5 | 2––– | 3
Carbon | C | 6 | 2–– | 4
Nitrogen | N | 7 | 2––– | 3
Oxygen | O | 8 | 2–– | 2
Fluorine | F | 9 | 2––– | 1
Neon | Ne | 10 | 2–– | 0
Sodium | Na | 11 | 2––– | 1
Magnesium | Mg | 12 | 2–– | 2
Aluminium | Al | 13 | 2––– | 3
Silicon | Si | 14 | 2–– | 4
Phosphorus | P | 15 | 2––– | 3 & 5
Sulphur | S | 16 | 2–– | 2
Chlorine | Cl | 17 | 2––– | 1
Argon | Ar | 18 | 2–– | 0
Valency
The electrons present in the outermost shell of an atom are known as the valence electrons. The electron configuration of an element describes how electrons are distributed in their atomic orbitals. They follow a standard notation in which all electron-containing atomic subshells are placed in a sequence. This method was suggested by Bohr and Bury.
The following rules are followed for writing the number of electrons in different energy levels or shells: The maximum number of electrons in a shell is given by the formula 2n2, where n is the orbit number i.e. 1,2,3 and so on. Therefore, the maximum number of electrons in different shells are as follows-
Orbit | Maximum Number of Electrons
K | 2
L | 8
M | 18
N | 32
Electrons are not accommodated in a given shell unless the inner shells are filled. That is, the shells are filled in a step-wise manner.
Why are electronic configurations useful?
Electron Configurations are useful for:
Determining the valency of an element.
Valency is the ability of an atom to form chemical bonds with other atoms. Valence electrons determine an element’s affinity to bond. When valence shells are full this usually is a good indicator that the electron is stable.
Predicting the properties of a group of elements.
The electronic configuration of an element can be used to predict the properties of a group of elements. For example, elements with similar electronic configurations tend to have similar chemical properties.
Interpreting atomic spectra.
Atomic spectra are the emission or absorption of light by atoms and molecules. The lines in the spectra are related to the energy difference between two electron configurations. By understanding the electronic configuration of an atom, we can interpret these spectra.
Electron configurations are an important tool for understanding the behavior of an atom and predicting the properties of a group of elements. By understanding how to find electrons per shell, the importance of electron configurations, and providing examples of electron configurations, we can better understand the properties of an atom.


Leave a Comment