Welcome to the wonderful world of atoms and their electron configurations!
Do you know what element has the electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3? If so, congratulations, you have taken the first step in understanding the basics of atomic structure!
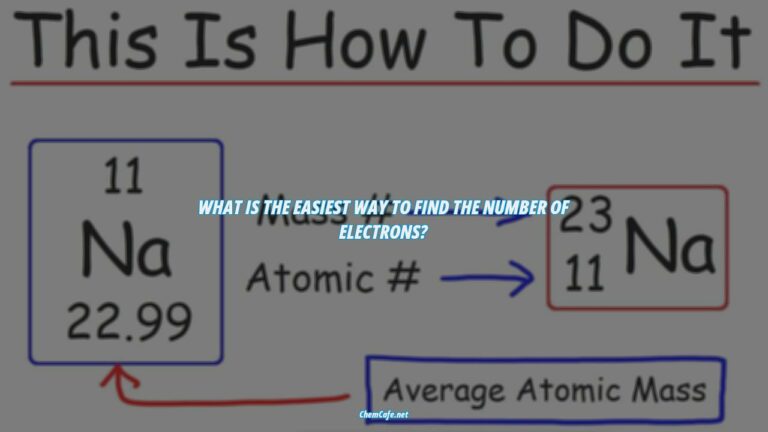
In chemistry, elements are defined by the number of protons they have in their nucleus. By counting up the number of electrons in a particular element, we can determine the element’s electron configuration. This configuration tells us a lot about the element, such as its group, atomic weight, and more.
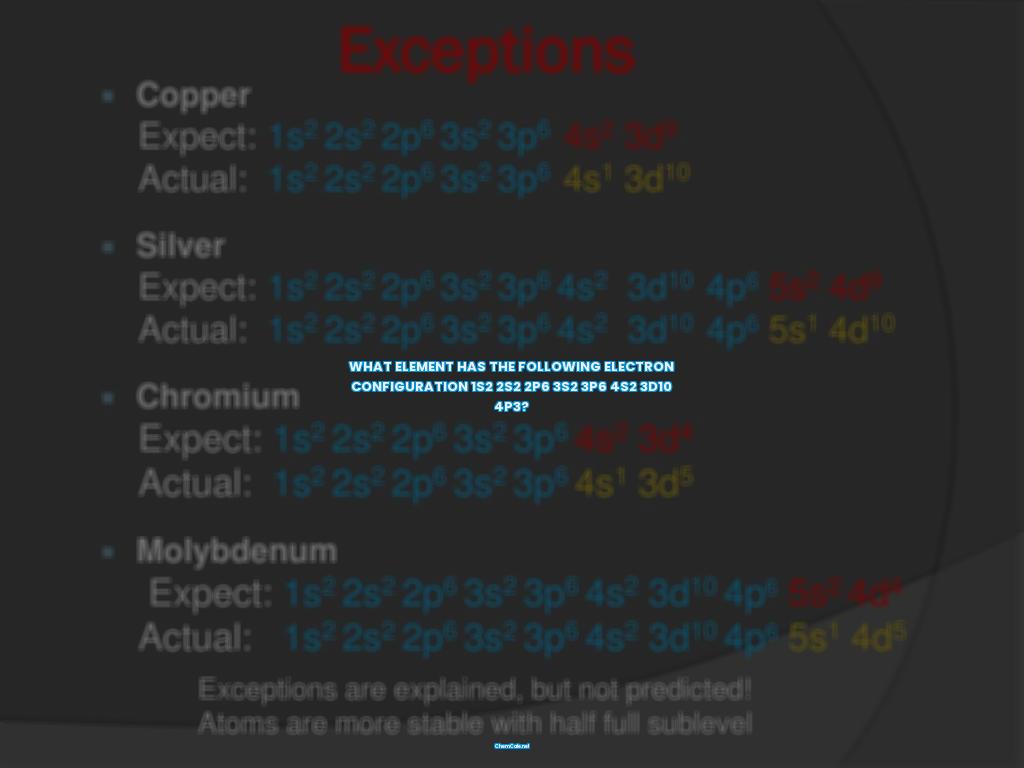
For example, a neutral atom of Vanadium (V) has the electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3. This tells us that Vanadium is part of the transition element group and has an atomic weight of 50.94.
And if we look at Iridium (Ir), we can see that it has the electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d7, which tells us that Iridium is also a transition element and has an atomic weight of 192.2.
As you can see, knowing the electron configuration of an element can be really useful! So now that you know what element has the electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3, you’re one step closer to understanding the basics of atomic structure and the different elements that make up the periodic table!
What element has the following electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3?
If you are looking for the element with the electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3, then you have come to the right place. This electron configuration is the signature of a neutral atom, meaning that the number of electrons is equal to the atomic number of the element. The element that has this electron configuration is Vanadium (V).
Vanadium is a transition element which is located in the fifth period of the periodic table. It has an atomic number of 23 and an atomic weight of 50.9415. This element is a silvery-grey metal that is naturally found in abundance in minerals such as carnotite, vanadinite and patronite.
Vanadium is a unique element that has several interesting properties. For example, it has the highest melting point of all the transition elements and is the only element that can exist in two oxidation states (+4 and +5). It is also the only element that can form a stable compound with oxygen, known as vanadium pentoxide.
Vanadium has many uses in industry. It is used to produce high-strength steel, which is stronger and more corrosion-resistant than ordinary steel. Vanadium is also used as an alloying agent in aluminum, titanium and other metals. In addition, it is used in the production of catalysts, batteries, fertilizers and dyes.
Vanadium has an important role in the human body, as it is essential for the proper functioning of enzymes. It is also believed to play a role in the regulation of blood sugar levels.
In conclusion, the element with the electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3 is Vanadium (V). This element is a transition metal that is found in abundance in minerals and has many interesting properties and uses. It is also important for the proper functioning of enzymes in the human body.
Which element has an electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3?
Atoms are the building blocks of matter, and the arrangement of electrons around an atom’s nucleus determines its chemical and physical properties. Each element on the periodic table has a unique electron configuration, which can be used to determine the element’s identity. So, which element has an electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3?
The answer is vanadium (V). Vanadium is a transition metal located in group 5 of the periodic table, and its atomic number is 23. This means that vanadium has 23 protons and 23 electrons in its neutral state. The 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3 electron configuration indicates that the 23 electrons of vanadium are arranged as follows: two in the 1s orbital, two in the 2s orbital, six in the 2p orbital, two in the 3s orbital, six in the 3p orbital, two in the 4s orbital, ten in the 3d orbital, and three in the 4p orbital.
Electron Configurations of Other Elements
It’s useful to compare the electron configuration of vanadium to the electron configurations of other elements. This can help you to better understand the arrangement of electrons in an atom’s outermost shells.
For example, iridium (Ir) is a transition metal located in group 9 of the periodic table. Its atomic number is 77, so it has 77 electrons in its neutral state. It has an electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d7. This indicates that the 77 electrons of iridium are arranged as follows: two in the 1s orbital, two in the 2s orbital, six in the 2p orbital, two in the 3s orbital, six in the 3p orbital, two in the 4s orbital, ten in the 3d orbital, six in the 4p orbital, two in the 5s orbital, ten in the 4d orbital, six in the 5p orbital, two in the 6s orbital, fourteen in the 4f orbital, seven in the 5d orbital.
Antimony (Sb) is a non-metal located in group 15 of the periodic table. Its atomic number is 51, so it has 51 electrons in its neutral state. It has an electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p3. This indicates that the 51 electrons of antimony are arranged as follows: two in the 1s orbital, two in the 2s orbital, six in the 2p orbital, two in the 3s orbital, six in the 3p orbital, two in the 4s orbital, ten in the 3d orbital, six in the 4p orbital, two in the 5s orbital, ten in the 4d orbital, and three in the 5p orbital.
Finally, tellurium (Te) is a transition metal located in group 16 of the periodic table. Its atomic number is 52, so it has 52 electrons in its neutral state. It has an electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p4. This indicates that the 52 electrons of tellurium are arranged as follows: two in the 1s orbital, two in the 2s orbital, six in the 2p orbital, two in the 3s orbital, six in the 3p orbital, two in the 4s orbital, ten in the 3d orbital, six in the 4p orbital, two in the 5s orbital, ten in the 4d orbital, and four in the 5p orbital.
To summarize, the element with an electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3 is vanadium (V). This electron configuration is unique to vanadium and can be used to identify it. However, other elements have similar electron configurations, such as iridium (Ir), antimony (Sb), and tellurium (Te). Knowing the electron configuration of each element can help to better understand their chemical and physical properties.
Which of the following elements has the electron configuration 1s2 2s2 2p6 3s2 3p1?
Electron configurations are an important part of understanding the behavior of atoms and molecules, as they determine the electronic and chemical properties of elements. Electron configurations are a way of organizing the electrons of an atom according to the energy levels they occupy. In short, they provide a way of visualizing the distribution of electrons in an atom.
What is an Electron Configuration?
An electron configuration is a notation that indicates the number of electrons in each energy level of an atom or ion. It is written using the symbol of the element and the superscripts indicating the number of electrons in each energy level. For example, the electron configuration of sodium (Na) is 1s22s22p63s1.
How are Electron Configurations Written?
Electron configurations are written in a standard notation, with the energy levels and subshells (each containing the number of electrons they hold written in superscript) written in a sequence. For example, the electron configuration of calcium (Ca) is 1s22s22p63s23p64s2.
Which of the following elements has the electron configuration 1s2 2s2 2p6 3s2 3p1?
The correct answer is element Mg (magnesium) with atomic number 12. The electron configuration of Mg is 1s2 2s2 2p6 3s2. The electron configuration of element Si (silicon) with atomic number 14 is 1s2 2s2 2p6 3s2 3p2. The electron configuration of element Na (sodium) with atomic number 11 is 1s2 2s2 2p6 3s1. The electron configuration of element Ne (neon) with atomic number 10 is 1s2 2s2 2p6. The electron configuration of element Al (aluminum) with atomic number 13 is 1s2 2s2 2p6 3s2 3p1.
Conclusion
In conclusion, the correct answer to the question “Which of the following elements has the electron configuration 1s2 2s2 2p6 3s2 3p1?” is element Mg (magnesium) with atomic number 12. Electron configurations are an important part of understanding the behavior of atoms and molecules. By understanding the electron configurations of elements, we can gain insight into their electronic and chemical properties.
Which element has the following electron configuration 1s2 2s2 2p6 3s2 3p6 4s1?
The electron configuration 1s2 2s2 2p6 3s2 3p6 4s1 corresponds to the element Magnesium (Mg). Magnesium is a chemical element with atomic number 12 and is located in the second group of the periodic table. This element is found in nature and is widely used in a variety of industries.
What are Electron Configurations?
Electron configurations are the arrangements of electrons around the nucleus of an atom according to the energy of the orbitals they are occupying. The standard notation for electron configurations involves placing all electron-containing atomic subshells in a sequence and indicating the number of electrons in each subshell with a superscript. For example, the electron configuration of sodium is 1s22s22p63s1.
Why are Electron Configurations Important?
Electron configurations are important for a variety of reasons. They allow us to determine the valency of an element, predict the properties of a group of elements (elements with similar electron configurations tend to exhibit similar properties), and interpret atomic spectra. This notation for the distribution of electrons in the atomic orbitals of atoms came into practice shortly after the Bohr model of the atom was presented by Ernest Rutherford and Niels Bohr in the year 1913.
Filling of Atomic Orbitals: Aufbau Principle
The Aufbau principle dictates that electrons will occupy the orbitals having lower energies before occupying higher energy orbitals. The energy of an orbital is calculated by the sum of the principal and the azimuthal quantum numbers.
The electron configuration 1s2 2s2 2p6 3s2 3p6 4s1 corresponds to the element Magnesium (Mg). Electron configurations are important for a variety of reasons, such as determining the valency of an element, predicting the properties of a group of elements, and interpreting atomic spectra. The Aufbau principle dictates that electrons will occupy the orbitals having lower energies before occupying higher energy orbitals.
What element has an electron configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1?
Atoms are made up of protons, neutrons, and electrons. Electrons are the smallest particles of an atom, and their configuration describes how they are arranged in an atom’s orbitals. The electron configuration of an element indicates the number of electrons located in each orbital and subshell of the atom.
The electron configuration of an element can be written in a shorthand notation or a standard notation. The standard notation follows the sequence of electron-containing atomic subshells and their corresponding superscripts, indicating the number of electrons in each subshell. For example, the electron configuration of sodium is 1s22s22p63s1.
What is the shorthand electron configuration for Pd?
Pd, or palladium, is a group 10, period 5 transition metal. Its electron configuration is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10. The shorthand version of this is [Kr] 4d10, since krypton’s electron configuration is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6.
Why are electron configurations important?
Electron configurations are important for a variety of reasons. They can be used to determine the valency of an element, which is the number of electrons available to form chemical bonds. Additionally, electron configurations can be used to predict the properties of a group of elements, since elements with similar electron configurations tend to exhibit similar properties.
Finally, electron configurations can be used to interpret atomic spectra. Atomic spectra are the visible light that is emitted when an atom is excited by an external energy source such as an electric current or light. By analyzing the wavelengths of the light emitted, scientists can determine the electron configurations of the atoms.
The electron configuration of an element describes how electrons are distributed in its atomic orbitals. The standard notation for electron configurations follows the sequence of electron-containing atomic subshells and their corresponding superscripts, indicating the number of electrons in each subshell. The shorthand electron configuration for Pd is [Kr] 4d10, since krypton’s electron configuration is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6. Electron configurations are important for determining the valency of an element, predicting the properties of a group of elements, and interpreting atomic spectra.
Which element has the following electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 quizlet?
Electron configurations are essential for understanding the structure and behavior of atoms, as well as the properties of elements. They are used to determine the valency of an atom, predict the properties of a group of elements, and interpret atomic spectra. But what exactly are electron configurations?
What are Electron Configurations?
Electron configurations are representations of the distribution of electrons in the atomic orbitals of atoms. The notation used to represent electron configurations follows a standard format, where all electron-containing atomic subshells (with the number of electrons they hold written in superscript) are placed in a sequence. For example, the electron configuration of sodium is 1s22s22p63s1.
How to Interpret Electron Configurations?
Interpreting electron configurations can be a bit tricky, especially for elements with a relatively large atomic number. The best way to understand them is to break them down into their component parts.
Let’s take the electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 as an example. This configuration can be broken down into five main parts:
1. The 1s orbital contains two electrons
2. The 2s orbital contains two electrons
3. The 2p orbital contains six electrons
4. The 3s orbital contains two electrons
5. The 3p orbital contains six electrons
6. The 4s orbital contains two electrons
7. The 3d orbital contains ten electrons
From this breakdown, we can see that the element represented by this electron configuration has a total of twenty-eight electrons. The element in question is Nickel (Ni).
What are Electron Configurations Used For?
Electron configurations are useful for a variety of applications. They can be used to determine the valency of an element, predict the properties of a group of elements (elements with similar electron configurations tend to exhibit similar properties), and interpret atomic spectra.
Conclusion
Electron configurations are an essential tool for understanding the structure and behavior of atoms and elements. By breaking down the configuration into its component parts, we can determine which element is represented by the configuration. In the example above, the electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 corresponds to Nickel (Ni).
Overall, electron configurations are useful for determining the valency of an element, predicting the properties of a group of elements, and interpreting atomic spectra. With a better understanding of electron configurations, we can gain a better understanding of the structure and behavior of atoms and elements.


Leave a Comment