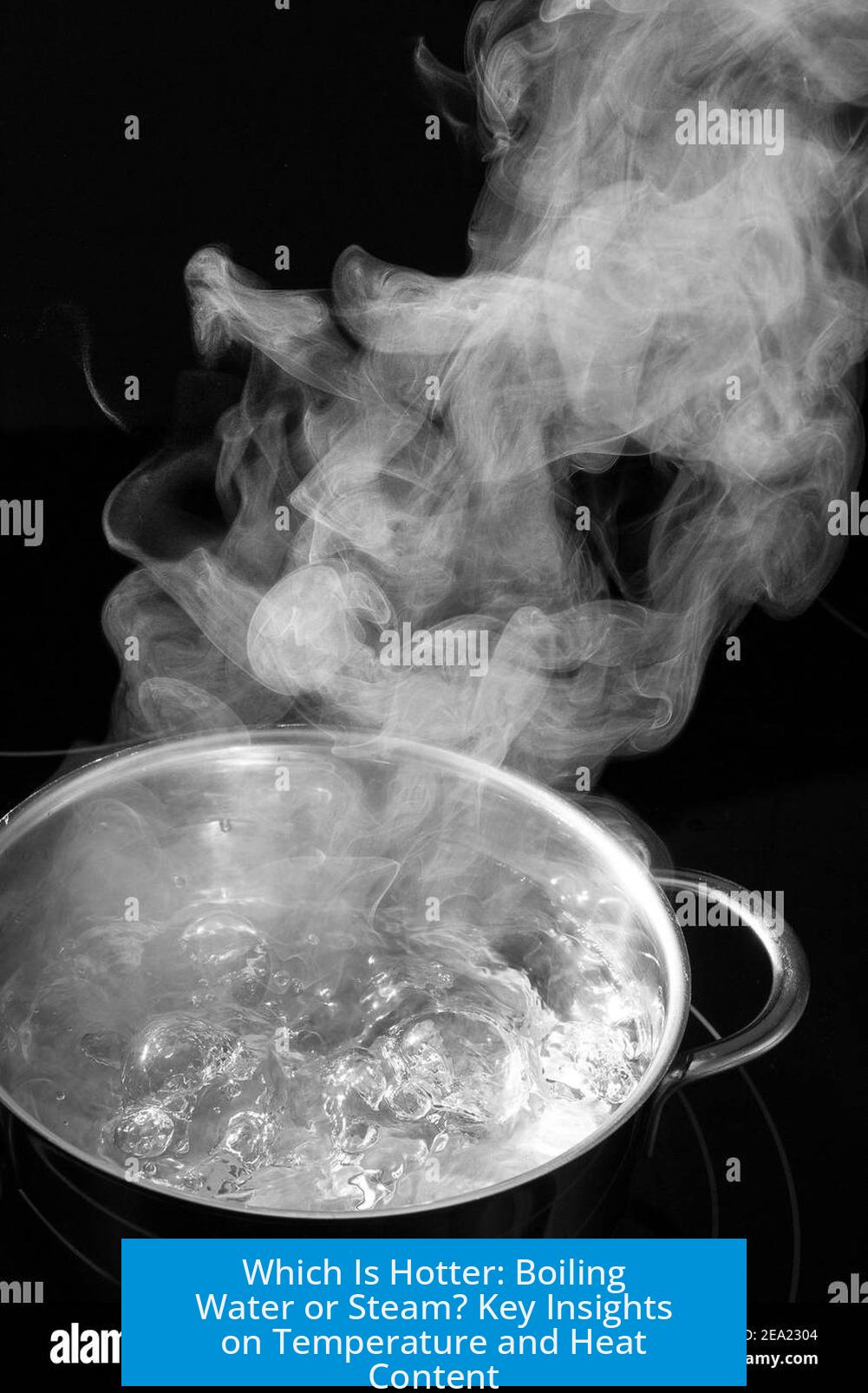
Which Is Hotter, Boiling Water or the Steam That Comes From It?
Steam that comes from boiling water is hotter in terms of heat energy, even though its temperature is the same as boiling water at the boiling point. Because steam contains latent heat from vaporization, it transfers more energy and can cause more severe burns than the liquid at 100 °C.
Temperature at the Boiling Point: Water and Steam
When water boils at sea level, it reaches its boiling point at approximately 100 °C (212 °F). At this temperature, water changes from the liquid state into steam, its gaseous state.
Critically, the steam produced directly above boiling water has the same temperature as the boiling water itself. This is because during the phase change from liquid to vapor, temperature remains constant. Energy added at this stage goes into breaking molecular bonds rather than raising temperature.
- Steam just formed at the surface is about 100 °C at one atmosphere pressure.
- The liquid water beneath is also at roughly 100 °C.
- Thus, when steam first leaves boiling water, its temperature equals that of the boiling water.
The equality of temperature means steam cannot be hotter in terms of degrees Celsius or Fahrenheit when it first forms. They represent the same thermal environment at the boiling point.
Heat Content: Why Steam Feels Hotter
While the temperature is the same for both phases at boiling, steam carries more energy per unit mass compared to boiling water. This extra energy comes from the latent heat of vaporization, the amount of heat required to transform water from liquid to vapor without changing temperature.
The latent heat of vaporization for water is about 2260 joules per gram (J/g) at 100 °C. This energy is stored in the steam as potential energy in the vapor phase and is released when steam condenses back to liquid.
Therefore:
- Steam at 100 °C contains both sensible heat (from its temperature) and latent heat.
- Boiling water contains only sensible heat at 100 °C.
- When steam contacts cooler skin, it condenses, releasing latent heat, delivering more energy than boiling water alone.
This explains why steam burns feel more severe—it transfers not only heat at 100 °C but also releases additional heat energy during condensation.
The Effect of Steam Condensation on Skin
When steam touches a cooler surface, such as skin:
- It condenses back into liquid water.
- The condensation releases latent heat stored in the steam.
- The resulting hot water remains at 100 °C, continuing heat transfer to the skin.
This dual heat delivery causes a “double dose” of thermal energy, making steam more dangerous than boiling water alone.
Can Steam Be Hotter Than Boiling Water?
Under typical conditions, steam and boiling water coexist at roughly the same temperature. However, steam can be heated beyond the boiling point under specific conditions:
- Superheated steam: Steam heated above 100 °C without liquid water present.
- This occurs when steam moves away from the boiling surface and absorbs extra heat.
- In controlled settings like industrial boilers or turbines, steam can reach temperatures of several hundred degrees Celsius.
- In common cases like a tea kettle, steam does not become superheated because it remains closely coupled with boiling water.
The Leidenfrost point demonstrates that vapor layers can insulate and allow superheated steam at a hot surface, but such conditions do not occur in everyday water boiling.
In practice:
- Steam from a typical boiling pot or kettle is not hotter than the water.
- Superheated steam requires special conditions absent in domestic boiling.
Factors Affecting the Temperatures of Steam and Boiling Water
Pressure Variations and Boiling Point Elevation
Pressure influences the boiling point. At higher pressures, water boils above 100 °C; at lower pressures, boiling occurs below 100 °C.
- In a large pot, pressure slightly increases at the bottom due to the weight of the water column.
- This causes a marginal temperature gradient from bottom to top of the liquid.
- Dissolved salts or impurities can elevate the boiling temperature slightly.
- Steam in the upper part tends to be purer and may have a slightly different temperature due to these factors.
Such variations are small but may cause minor temperature differences between steam and liquid water inside a container.
Heat Distribution and Cooling Effects
Uneven heating and cooling play roles in variations of temperatures for both steam and water:
- Parts of a container’s liquid may be hotter than others.
- Cooling around container edges lowers temperature locally.
- Steam leaving an opening cools gradually as it mixes with ambient air.
- Thus, steam at the mouth of a teacup may be cooler than the liquid inside.
Overall, these environmental factors affect practical observations but do not change the fundamental thermal properties of steam and water at boiling.
Summary of Key Points
- Temperature equality: Boiling water and the steam just above it share the same temperature, about 100 °C at sea level pressure.
- Latent heat content: Steam contains extra heat energy from vaporization, which makes it feel hotter despite having the same temperature as water.
- Steam burns: Steam is more dangerous because it delivers heat twice—during condensation and as hot water afterward.
- Superheated steam: Steam can exceed 100 °C in specialized conditions, but this does not occur in everyday water boiling.
- Environmental effects: Pressure differences, impurities, and uneven heating cause minor temperature variations between steam and boiling water.


Leave a Comment