Why Does This Reaction Add a Tert-Butyl Group Instead of an Isobutyl Group?
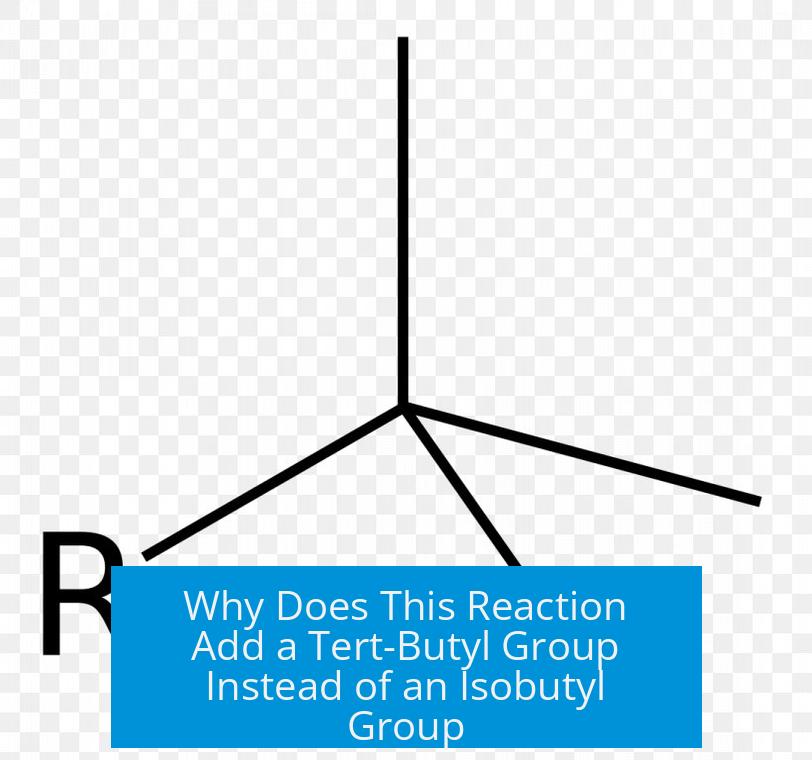
The reaction adds a tert-butyl group instead of an isobutyl group because the initially formed primary carbocation rearranges via a 1,2-hydride shift into a more stable tertiary carbocation, which then reacts to give the tert-butyl substituted product.
Carbocation Formation and Rearrangement
At the start, the alkyl halide reacts in a way that forms a primary carbocation. Primary carbocations are unstable and rarely exist long enough to react directly. The reaction mechanism prompts this carbocation to rearrange rapidly.
The rearrangement occurs through a 1,2-hydride shift. This shift means that a hydrogen atom with its bonding electrons moves from an adjacent carbon to the carbocation center. Such a shift changes the carbocation from primary to tertiary.
Why Tertiary Carbocation Stability Matters
- Tertiary carbocations have three alkyl groups donating electron density, stabilizing the positive charge effectively.
- Secondary and primary carbocations do not stabilize the charge as well, making tertiary carbocations favored intermediates.
- Rearrangement to the tertiary carbocation ensures the reaction proceeds via the most stable carbocation intermediate.
Impact on the Final Product
Once the stable tertiary carbocation forms, it participates in the Friedel-Crafts alkylation. This reaction introduces the alkyl group onto an aromatic ring.
Due to the rearrangement, the carbocation structure corresponds to the tert-butyl group, not the isobutyl group. In other words, the alkyl group attached to the ring reflects the tertiary carbocation structure, leading to tert-butyl substitution.
Summary Table of the Process
| Step | Description |
|---|---|
| 1 | Primary carbocation forms initially |
| 2 | 1,2-hydride shift causes rearrangement |
| 3 | Tertiary carbocation forms (more stable) |
| 4 | Tertiary carbocation reacts in Friedel-Crafts alkylation |
| 5 | Final product has tert-butyl group, not isobutyl |
Key Takeaways
- This reaction relies on carbocation rearrangement to stabilize intermediates.
- 1,2-Hydride shifts convert unstable primary carbocations into tertiary carbocations.
- Tertiary carbocations lead to tert-butyl substitution, not isobutyl.
- Friedel-Crafts alkylation proceeds via the most stable carbocation intermediate.
- Reaction outcome depends on carbocation stability and rearrangement possibilities.
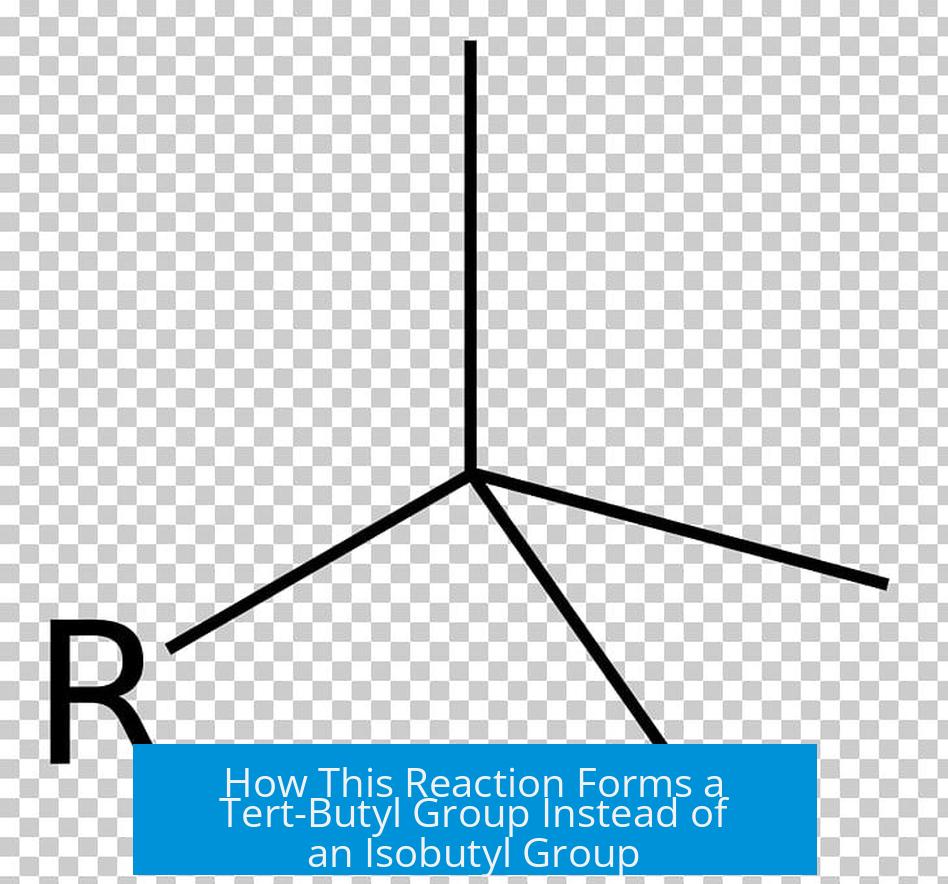
Why does the reaction form a tert-butyl group instead of an isobutyl group?
The reaction forms a primary carbocation, which is unstable. It quickly rearranges via a 1,2-hydride shift to a stable tertiary carbocation, leading to the tert-butyl group rather than the isobutyl group.
What role does the 1,2-hydride shift play in this reaction?
The 1,2-hydride shift moves a hydride ion to the carbocation center. This shift converts a less stable primary carbocation into a more stable tertiary carbocation, directing the product to have a tert-butyl group.
How does carbocation stability affect the product formed?
Tertiary carbocations are more stable than primary or secondary ones. The reaction favors the formation of the stable tertiary carbocation, which results in attaching a tert-butyl group instead of an isobutyl group.
Why is carbocation rearrangement common in Friedel-Crafts alkylation?
Friedel-Crafts alkylation often forms carbocation intermediates. These intermediates rearrange to more stable forms to lower the reaction’s energy. This rearrangement influences the alkyl group that adds to the aromatic ring.


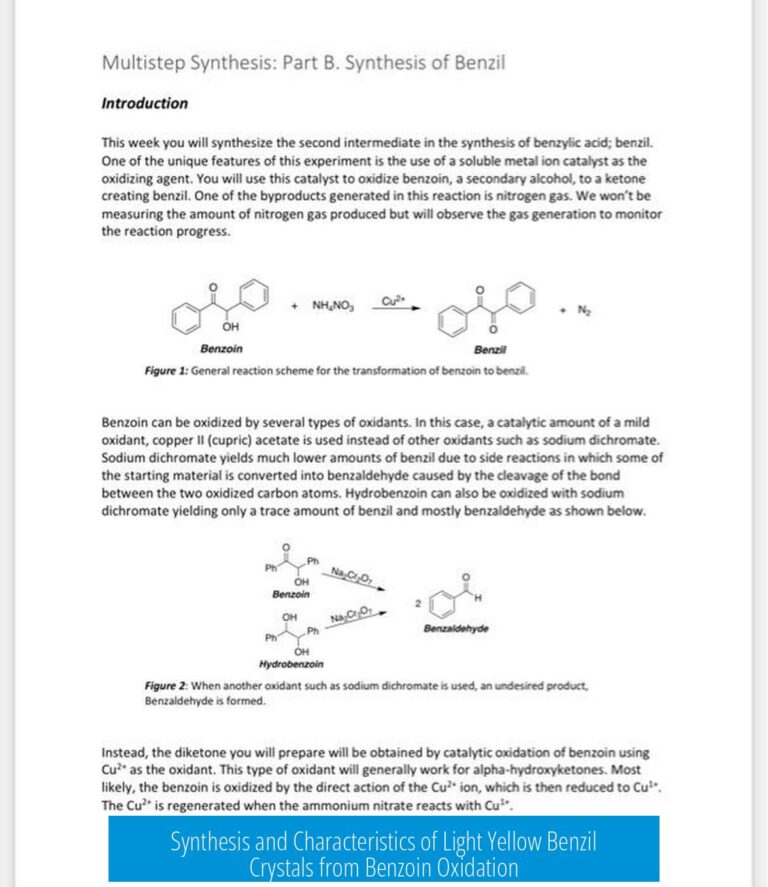

Leave a Comment