Grignard Reagent vs Gilman Reagent
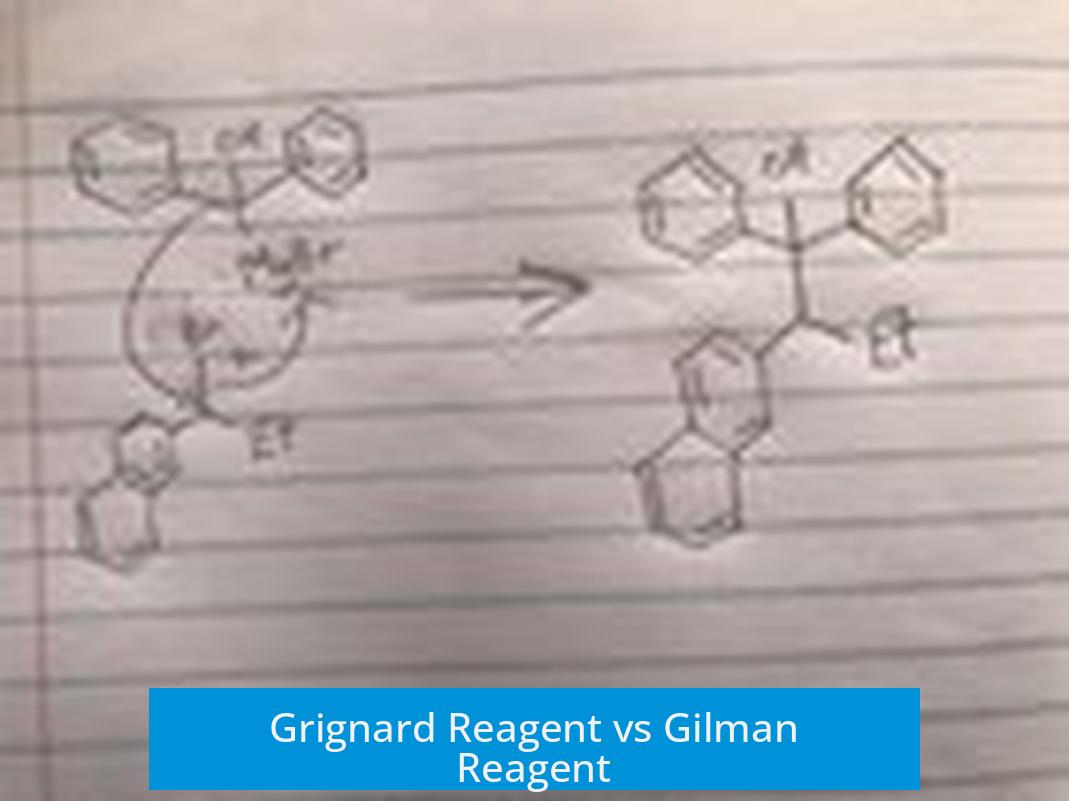
Grignard reagents and Gilman reagents differ mainly in nucleophilicity, selectivity, and reactivity, influencing how each behaves in organic synthesis.
1. Nucleophilicity and Selectivity
Gilman reagents act as softer nucleophiles compared to Grignard reagents. This softness enhances their selectivity, especially in conjugate additions. In conjugated systems, Grignard reagents favor 1,2-addition due to electrostatic attractions. In contrast, Gilman reagents prefer 1,4-addition driven by orbital interactions.
This difference is critical in designing synthetic routes, allowing chemists to target either direct addition to the carbonyl or conjugate sites more precisely.
2. Reactivity Differences
Gilman reagents are milder than Grignard reagents. This milder nature reflects in their interaction with sensitive electrophiles. For example, with acid chlorides:
- Grignard reagents add twice, ultimately forming alcohols via intermediate ketones.
- Gilman reagents add once, stopping at the ketone stage.
This controlled reactivity makes Gilman reagents preferable when over-addition must be avoided, preserving functional group integrity.
3. Applications and Reaction Preferences
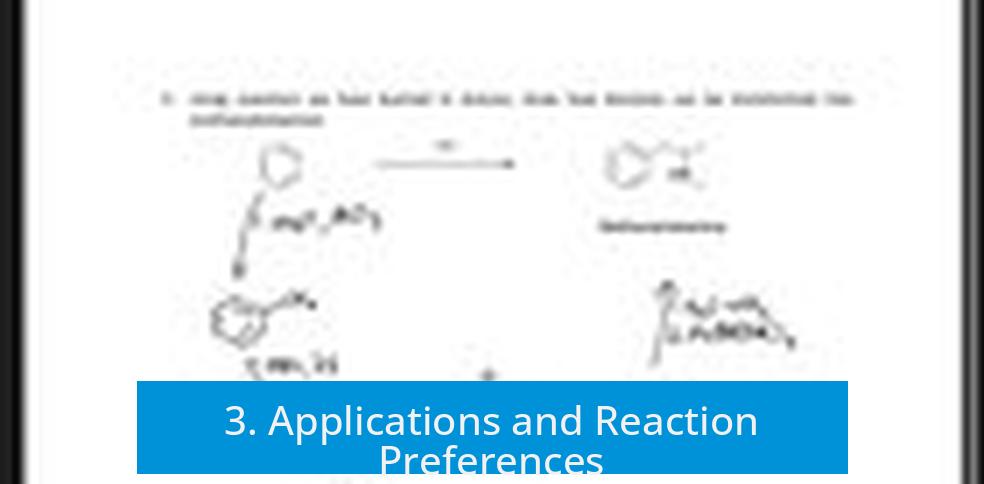
Gilman reagents (organocuprates) excel in soft 1,4-additions to π-systems such as α,β-unsaturated carbonyl compounds. Their selectivity and mildness make them suited for such transformations.
Additionally, Gilman reagents exhibit better performance in SN2 reactions, such as alkylation involving bromine substituents, compared to Grignard reagents. This preference influences the choice of reagent in substitution reactions requiring precision.
| Property | Grignard Reagent | Gilman Reagent |
|---|---|---|
| Nucleophile Type | Hard nucleophile | Softer nucleophile |
| Conjugate Addition | 1,2-addition favored | 1,4-addition favored |
| Reactivity with Acid Chlorides | Double addition → Alcohols | Single addition → Ketones |
| Suitability for SN2 | Less effective | More effective |
Key Takeaways
- Gilman reagents are softer nucleophiles, allowing selective 1,4-addition in conjugated systems.
- Grignard reagents are more reactive, often causing multiple additions, especially with acid chlorides.
- Gilman reagents are milder and better suited for reactions requiring controlled single additions.
- Gilman reagents outperform Grignards in SN2 reactions and soft alkylations.





Leave a Comment