Trouble with NBS Bromination
NBS bromination is a robust yet occasionally challenging reaction. Commonly conducted in carbon tetrachloride, it is characterized by the slow disappearance of NBS at the flask’s bottom and gradual accumulation of succinimide at the top of the solution. Understanding the reaction’s visual cues and procedural nuances helps resolve common issues.
Reaction Characteristics and Monitoring

- The reaction is often called the “up and down reaction” due to the movement of reagents within carbon tetrachloride.
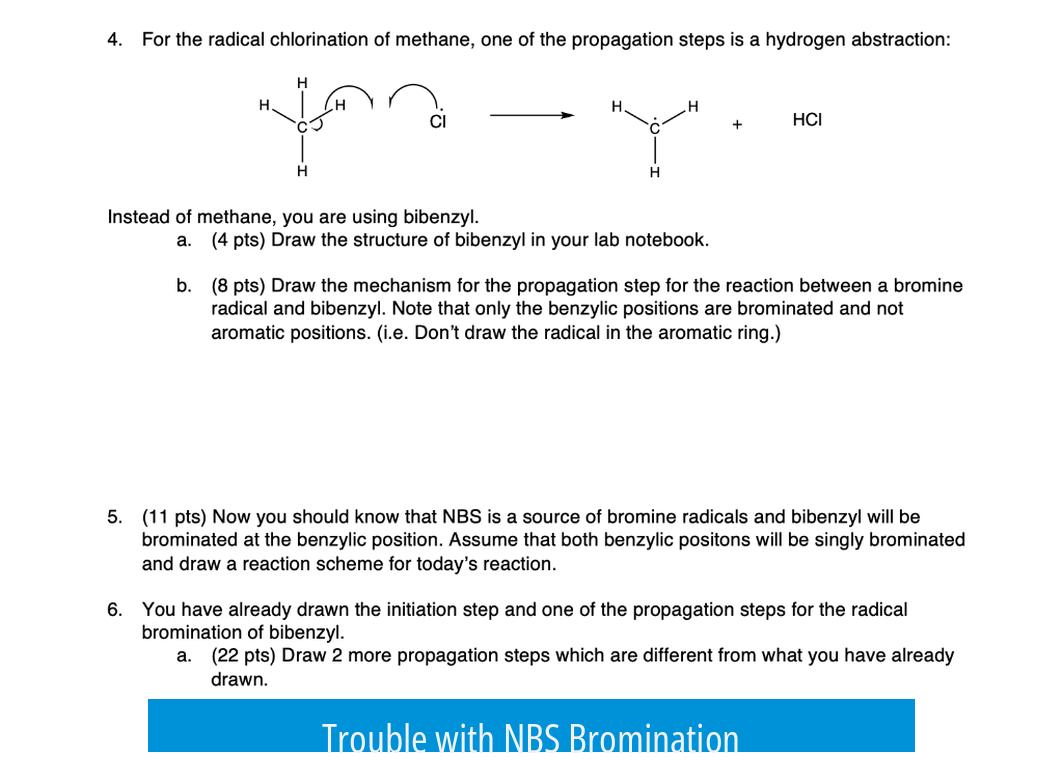
- Progress is easily monitored visually as NBS disappears and succinimide forms.
- A typical positive indication includes observing a deep orange or brown color, indicating bromine formation.
Procedural Guidance
Sticking closely to established procedures, such as those documented in Organic Syntheses, is crucial. These protocols are reliable and usually “bulletproof.”
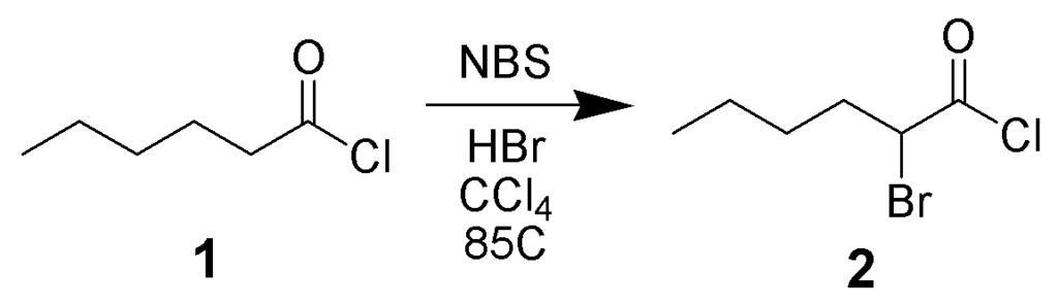
Key factors to check include:
- NBS consumption: Ensure NBS is being consumed as the reaction proceeds.
- Light source: Use an incandescent light since it supports radical bromination effectively.
- Additives: A drop of aqueous HBr can enhance reaction progress if it stalls.
- Visual signs: Monitor for bromine formation, which appears as an intense orange/brown tint.
Common Problems and Troubleshooting
| Issue | Possible Cause | Suggested Action |
|---|---|---|
| Slow or no reaction progress | Impure or degraded carbon tetrachloride | Redistill carbon tetrachloride to improve quality |
| No bromine formation | Improper light source or lack of initiator | Use incandescent lamp and consider adding aqueous HBr |
| Inconsistent or no NBS consumption | Contaminated or low purity NBS | Check NBS by NMR analysis to confirm purity |
| Unexpected results or no product | Substrate incompatibility or impurity | Test reaction with a well-known substrate such as indanone |
Solvent dryness and ultra-purity are usually not critical, as the reaction tolerates reasonable impurity levels well.
Summary of Key Points
- The “up and down reaction” refers to NBS bromination in carbon tetrachloride.
- Progress can be monitored visually by NBS disappearance and succinimide formation.
- Use incandescent light and consider aqueous HBr to promote reaction.
- Redistill carbon tetrachloride if reaction slows or stalls.
- Verify NBS purity by NMR to exclude contaminants.
- Try different substrates to rule out substrate issues.
The Trouble with NBS Bromination: What’s Really Going On?
If you’re wrestling with NBS bromination and wondering why your reaction refuses to behave, you’re not alone. The process is well-known, but things can get tricky fast. So here’s the bottom line: NBS bromination is usually reliable, but small hiccups in your reagents or conditions can cause the dreaded “up and down” reaction, incomplete bromine formation, or puzzling reaction stalls.
Let’s unpack these common problems, offer practical fixes, and illuminate the path to a clean bromination reaction.
What Exactly is the “Up and Down” Reaction?
This quirky nickname refers to the behavior seen when running an NBS bromination in carbon tetrachloride (CCl4). You literally see the NBS sinking to the bottom of the flask and slowly disappearing. Meanwhile, its byproduct, succinimide, climbs to the top, showing you visual proof that the reaction is crawling along.
Think of it like a tiny chemical elevator.
Does this visual cue tell you something straightforward? Yes: the reaction is happening, but patience is your friend.
Sticking to Proven Procedures Is Key
Organic Syntheses (.Org Syn) procedures are generally bulletproof for NBS bromination. So, if your reaction’s misbehaving, first ask: Am I following their exact protocol?
Even slight tweaks can derail a delicate balance. Ensure you’re using the exact solvent, light source, and addition sequences.
Is Your NBS Actually Being Consumed?
This is the simplest checkpoint often missed. If the NBS powder stubbornly sticks around at the bottom of the flask, the reaction probably isn’t progressing as it should. Tracking NBS consumption is a direct window into your reaction’s health.
Still wondering why it’s not disappearing? That leads to our next point.
Lighting Matters: Incandescent vs. Other Sources
Ever thought about the type of light shining on your reaction? It influences radical bromination notably. Incandescent light gently encourages the homolytic cleavage needed without overwhelming energy that might overdo side reactions.
Fluorescent or LED lights might not cut it—so switch to an incandescent bulb if your reaction feels sluggish.
Sometimes A Drop of HBr Makes a Big Difference
Yes, it sounds odd adding an acid to a radical bromination, but a tiny splash of aqueous hydrobromic acid can nudge the reaction forward.
It seems to help generate active bromine radicals by facilitating bromine formation in solution. If your reaction isn’t forming that classic deep orange/brown bromine color, try this tweak before tossing everything out.
Do You See that Deep Orange/Brown Bromine Color?
Bromine formation is your reaction’s neon sign. When you don’t see it, your bromination is probably stuck in neutral.
Remember: In ideal conditions, your flask should glow with a deep amber hue. No color? Something’s missing.
Quality of Carbon Tetrachloride: The Silent Culprit
If your solvent is compromised, the entire reaction can stall. Carbon tetrachloride’s purity directly impacts radical initiations and bromine solubility.
Guess what? Distilling your CCl4 freshly is often a quick fix.
Don’t be shy about this: contaminated solvents throw a wrench in the works more than slightly damp or impure organic compounds.
Stop Worrying About Ultra-Pure Solvents
This reaction doesn’t freak out over trace water or minor impurities. NBS bromination is robust in that way.
So, obsessive drying isn’t necessary here. Focus your energy where it truly matters—the substrate, solvent quality, and light source.
Could Your Substrate Be the Villain?
Sometimes the molecule you want to brominate is the problem. If the reaction falters, test a trusted compound like indanone instead.
This step eliminates mystery and directs troubleshooting:
- If indanone brominates smoothly, your original substrate might be inert or incompatible
- If the indanone test also fails, look at your reagents or conditions
Is Your NBS Pure Enough?
Don’t overlook NBS purity. NMR analysis can reveal nasty surprises like contamination or degradation.
Your supposedly powerful brominating agent might be a dud if it’s crammed with impurities.
Summary and Quick Checks for Success
- Follow literature protocols exactly. Especially .Org Syn methods.
- Check NBS consumption visually and by NMR.
- Use incandescent light. It helps radical formation.
- Look for the bromine color. Deep orange/brown means good progress.
- Consider adding a drop of aqueous HBr. It kickstarts radical generation.
- Redistill carbon tetrachloride. Quality solvent is often the hidden key.
- Don’t obsess over ultra-pure solvents. This reaction generally tolerates some impurities.
- Test another substrate. Like indanone, to confirm your setup.
- Verify NBS purity. With NMR to rule out contaminants.
Final Thoughts
The “trouble with NBS bromination” usually boils down to small but impactful details. It’s not rocket science but requires a bit of forensic chemistry detective work.
Worried your experiment is doomed? Look again. Most failures stem from solvable issues: the light bulb you’re using, if your carbon tet is stale, or if your substrates and reagents are up to snuff.
Remember, NBS bromination is a classic for a reason. Stick to verified methods, monitor visually, and tweak systematically.
Next time your NBS appears stubborn, channel your inner Sherlock: Is your NBS really disappearing? Is the bromine color vivid? Are you following the Org Syn gospel?
Fix these, and your bromination will turn from “up and down” blues into a smooth “success” story.
Why does the NBS bromination reaction show an “up and down” behavior?
This occurs when using carbon tetrachloride as the solvent. NBS settles at the bottom, slowly disappears, while succinimide forms at the top of the solution. The visual change helps monitor the reaction progress.
How can I tell if my NBS bromination is proceeding correctly?
Watch for the disappearance of NBS and formation of succinimide. Also, check if bromine forms, usually causing a deep orange or brown color in the solution. If not, the reaction might be stalled.
What steps can I take if my NBS bromination is not working as expected?
Try redistilling your carbon tetrachloride solvent and check the purity of your NBS by NMR. Consider testing the reaction with a different substrate like indanone to rule out substrate issues.
Is it necessary to use ultra-pure, dry solvents for NBS bromination?
No, this reaction is quite robust. Obsessing over solvent purity or dryness is usually unnecessary. Focus more on the solvent’s quality and the NBS condition.
Can additives improve the NBS bromination reaction?
Sometimes adding a drop of aqueous HBr helps initiate or speed up the reaction. Also, using incandescent light instead of other light sources might influence the reaction progress.



Leave a Comment